Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency
- PMID: 23531552
- PMCID: PMC3666632
- DOI: 10.1038/mt.2013.34
Long-term follow-up of foamy viral vector-mediated gene therapy for canine leukocyte adhesion deficiency
Abstract
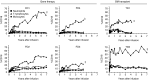
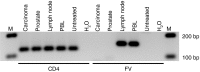
The development of leukemia following gammaretroviral vector-mediated gene therapy for X-linked severe combined immunodeficiency disease and chronic granulomatous disease (CGD) has emphasized the need for long-term follow-up in animals treated with hematopoietic stem cell gene therapy. In this study, we report the long-term follow-up (4-7 years) of four dogs with canine leukocyte adhesion deficiency (CLAD) treated with foamy viral (FV) vector-mediated gene therapy. All four CLAD dogs previously received nonmyeloablative conditioning with 200 cGy total body irradiation followed by infusion of autologous, CD34(+) hematopoietic stem cells transduced by a FV vector expressing canine CD18 from an internal Murine Stem Cell Virus (MSCV) promoter. CD18(+) leukocyte levels were >2% following infusion of vector-transduced cells leading to ongoing reversal of the CLAD phenotype for >4 years. There was no clinical development of lymphoid or myeloid leukemia in any of the four dogs and integration site analysis did not reveal insertional oncogenesis. These results showing disease correction/amelioration of disease in CLAD without significant adverse events provide support for the use of a FV vector to treat children with leukocyte adhesion deficiency type 1 (LAD-1) in a human gene therapy clinical trial.
Figures




Similar articles
-
Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors.Nat Med. 2008 Jan;14(1):93-7. doi: 10.1038/nm1695. Epub 2007 Dec 23. Nat Med. 2008. PMID: 18157138 Free PMC article.
-
Treatment of canine leukocyte adhesion deficiency by foamy virus vectors expressing CD18 from a PGK promoter.Gene Ther. 2011 Jun;18(6):553-9. doi: 10.1038/gt.2010.169. Epub 2011 Jan 13. Gene Ther. 2011. PMID: 21228879 Free PMC article.
-
Gene therapy of canine leukocyte adhesion deficiency using lentiviral vectors with human CD11b and CD18 promoters driving canine CD18 expression.Mol Ther. 2011 Jan;19(1):113-21. doi: 10.1038/mt.2010.203. Epub 2010 Sep 21. Mol Ther. 2011. PMID: 20859258 Free PMC article.
-
Large animal models for foamy virus vector gene therapy.Viruses. 2012 Dec 7;4(12):3572-88. doi: 10.3390/v4123572. Viruses. 2012. PMID: 23223198 Free PMC article. Review.
-
Leukocyte adhesion deficiency in children and Irish setter dogs.Pediatr Res. 2004 Mar;55(3):363-7. doi: 10.1203/01.PDR.0000111287.74989.1B. Epub 2004 Jan 7. Pediatr Res. 2004. PMID: 14711903 Review.
Cited by
-
Periodontal and other oral manifestations of immunodeficiency diseases.Oral Dis. 2017 Oct;23(7):866-888. doi: 10.1111/odi.12584. Epub 2016 Oct 10. Oral Dis. 2017. PMID: 27630012 Free PMC article. Review.
-
Impact of gene therapy for canine monogenic diseases on the progress of preclinical studies.J Appl Genet. 2020 May;61(2):179-186. doi: 10.1007/s13353-020-00554-8. Epub 2020 Mar 18. J Appl Genet. 2020. PMID: 32189222 Free PMC article. Review.
-
Foamy virus vectors for HIV gene therapy.Viruses. 2013 Oct 22;5(10):2585-600. doi: 10.3390/v5102585. Viruses. 2013. PMID: 24153061 Free PMC article. Review.
-
Contemporary Animal Models For Human Gene Therapy Applications.Curr Gene Ther. 2015;15(6):531-40. doi: 10.2174/1566523215666150929110424. Curr Gene Ther. 2015. PMID: 26415576 Free PMC article. Review.
-
Eleventh International Foamy Virus Conference-Meeting Report.Viruses. 2016 Nov 23;8(11):318. doi: 10.3390/v8110318. Viruses. 2016. PMID: 27886074 Free PMC article.
References
-
- Morianos I, Siapati EK, Pongas G., and, Vassilopoulos G. Comparative analysis of FV vectors with human α- or β-globin gene regulatory elements for the correction of β-thalassemia. Gene Ther. 2012;19:303–311. - PubMed
-
- Chatziandreou I, Siapati EK., and, Vassilopoulos G. Genetic correction of X-linked chronic granulomatous disease with novel foamy virus vectors. Exp Hematol. 2011;39:643–652. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

