ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination
- PMID: 23531630
- PMCID: PMC3607800
- DOI: 10.3389/fpls.2013.00063
ABA crosstalk with ethylene and nitric oxide in seed dormancy and germination
Abstract
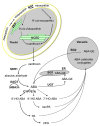
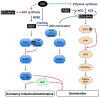
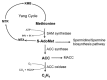
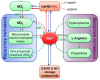
Dormancy is an adaptive trait that enables seed germination to coincide with favorable environmental conditions. It has been clearly demonstrated that dormancy is induced by abscisic acid (ABA) during seed development on the mother plant. After seed dispersal, germination is preceded by a decline in ABA in imbibed seeds, which results from ABA catabolism through 8'-hydroxylation. The hormonal balance between ABA and gibberellins (GAs) has been shown to act as an integrator of environmental cues to maintain dormancy or activate germination. The interplay of ABA with other endogenous signals is however less documented. In numerous species, ethylene counteracts ABA signaling pathways and induces germination. In Brassicaceae seeds, ethylene prevents the inhibitory effects of ABA on endosperm cap weakening, thereby facilitating endosperm rupture and radicle emergence. Moreover, enhanced seed dormancy in Arabidopsis ethylene-insensitive mutants results from greater ABA sensitivity. Conversely, ABA limits ethylene action by down-regulating its biosynthesis. Nitric oxide (NO) has been proposed as a common actor in the ABA and ethylene crosstalk in seed. Indeed, convergent evidence indicates that NO is produced rapidly after seed imbibition and promotes germination by inducing the expression of the ABA 8'-hydroxylase gene, CYP707A2, and stimulating ethylene production. The role of NO and other nitrogen-containing compounds, such as nitrate, in seed dormancy breakage and germination stimulation has been reported in several species. This review will describe our current knowledge of ABA crosstalk with ethylene and NO, both volatile compounds that have been shown to counteract ABA action in seeds and to improve dormancy release and germination.
Keywords: abscisic acid; dormancy; ethylene; germination; hormone; nitric oxide; seed.
Figures
References
-
- Abat J. K., Mattoo A. K., Deswal R. (2008). S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata – ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J. 275 2862–2872 - PubMed
-
- Alboresi A., Gestin C., Leydecker M. T., Bedu M., Meyer C., Truong H. N. (2005). Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 28 500–512 - PubMed
-
- Alonso J. M., Hirayama T., Roman G., Nourizadeh S., Ecker J. R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

