Expression of Pneumocystis jirovecii major surface glycoprotein in Saccharomyces cerevisiae
- PMID: 23532098
- PMCID: PMC3666137
- DOI: 10.1093/infdis/jit131
Expression of Pneumocystis jirovecii major surface glycoprotein in Saccharomyces cerevisiae
Abstract
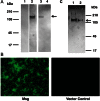
The major surface glycoprotein (Msg), which is the most abundant protein expressed on the cell surface of Pneumocystis organisms, plays an important role in the attachment of this organism to epithelial cells and macrophages. In the present study, we expressed Pneumocystis jirovecii Msg in Saccharomyces cerevisiae, a phylogenetically related organism. Full-length P. jirovecii Msg was expressed with a DNA construct that used codons optimized for expression in yeast. Unlike in Pneumocystis organisms, recombinant Msg localized to the plasma membrane of yeast rather than to the cell wall. Msg expression was targeted to the yeast cell wall by replacing its signal peptide, serine-threonine-rich region, and glycophosphatidylinositol anchor signal region with the signal peptide of cell wall protein α-agglutinin of S. cerevisiae, the serine-threonine-rich region of epithelial adhesin (Epa1) of Candida glabrata, and the carboxyl region of the cell wall protein (Cwp2) of S. cerevisiae, respectively. Immunofluorescence analysis and treatment with β-1,3 glucanase demonstrated that the expressed Msg fusion protein localized to the yeast cell wall. Surface expression of Msg protein resulted in increased adherence of yeast to A549 alveolar epithelial cells. Heterologous expression of Msg in yeast will facilitate studies of the biologic properties of Pneumocystis Msg.
Keywords: GPI anchored protein; Pneumocystis jirovecii; antigenic variation; major surface glycoprotein; upstream conserved sequence.
Figures




Similar articles
-
Characterization of pneumocystis major surface glycoprotein gene (msg) promoter activity in Saccharomyces cerevisiae.Eukaryot Cell. 2013 Oct;12(10):1349-55. doi: 10.1128/EC.00122-13. Epub 2013 Jul 26. Eukaryot Cell. 2013. PMID: 23893080 Free PMC article.
-
Pneumocystis carinii Major Surface Glycoprotein Dampens Macrophage Inflammatory Responses to Fungal β-Glucan.J Infect Dis. 2020 Sep 1;222(7):1213-1221. doi: 10.1093/infdis/jiaa218. J Infect Dis. 2020. PMID: 32363390 Free PMC article.
-
Variation in the major surface glycoprotein genes in Pneumocystis jirovecii.J Infect Dis. 2008 Sep 1;198(5):741-9. doi: 10.1086/590433. J Infect Dis. 2008. PMID: 18627244 Free PMC article.
-
Characterization of the expression site of the major surface glycoprotein of human-derived Pneumocystis carinii.Mol Microbiol. 2001 Oct;42(1):183-93. doi: 10.1046/j.1365-2958.2001.02620.x. Mol Microbiol. 2001. PMID: 11679077
-
Antigenic variation in pneumocystis.J Eukaryot Microbiol. 2007 Jan-Feb;54(1):8-13. doi: 10.1111/j.1550-7408.2006.00225.x. J Eukaryot Microbiol. 2007. PMID: 17300510 Review.
Cited by
-
Evidence of the Red-Queen Hypothesis from Accelerated Rates of Evolution of Genes Involved in Biotic Interactions in Pneumocystis.Genome Biol Evol. 2018 Jun 1;10(6):1596-1606. doi: 10.1093/gbe/evy116. Genome Biol Evol. 2018. PMID: 29893833 Free PMC article.
-
Host cell invasion by medically important fungi.Cold Spring Harb Perspect Med. 2014 Nov 3;5(1):a019687. doi: 10.1101/cshperspect.a019687. Cold Spring Harb Perspect Med. 2014. PMID: 25367974 Free PMC article. Review.
-
Lung Epithelial Cell Line Immune Responses to Pneumocystis.J Fungi (Basel). 2023 Jul 6;9(7):729. doi: 10.3390/jof9070729. J Fungi (Basel). 2023. PMID: 37504718 Free PMC article. Review.
-
Adhesins in the virulence of opportunistic fungal pathogens of human.Mycology. 2021 Jul 5;12(4):296-324. doi: 10.1080/21501203.2021.1934176. eCollection 2021. Mycology. 2021. PMID: 34900383 Free PMC article. Review.
-
What We Do Not Know about Fungal Cell Adhesion Molecules.J Fungi (Basel). 2018 May 17;4(2):59. doi: 10.3390/jof4020059. J Fungi (Basel). 2018. PMID: 29772751 Free PMC article. Review.
References
-
- Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009;301:2578–85. - PubMed
-
- Thomas CF, Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350:2487–98. - PubMed
-
- Stringer JR, Stringer SL, Zhang J, Baughman R, Smulian AG, Cushion MT. Molecular genetic distinction of Pneumocystis carinii from rats and humans. J Eukaryot Microbiol. 1993;40:733–41. - PubMed
-
- Sinclair K, Wakefield AE, Banerji S, Hopkin JM. Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol Biochem Parasitol. 1991;45:183–4. - PubMed
-
- Mazars E, Guyot K, Durand I, et al. Isoenzyme diversity in Pneumocystis carinii from rats, mice, and rabbits. J Infect Dis. 1997;175:655–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

