IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells
- PMID: 23532711
- PMCID: PMC3607237
- DOI: 10.7150/ijms.5325
IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells
Abstract
Background: Long-term maintenance of neural stem cells in vitro is crucial for their stage specific roles in neurogenesis. To have an in-depth understanding of optimal conditional microenvironmental niche for long-term maintenance of neural stem cells (NSCs), we imposed different combinatorial treatment of growth factors to EGF/FGF-responsive cells. We hypothesized, that IGF-1-treatment can provide an optimal niche for long-term maintenance and proliferation of EGF/FGF-responsive NSCs.
Objective: This study was performed to investigate the cellular morphology and growth of rat embryonic striatal tissue derived-NSCs in long-term culture under the influence of different combinatorial effects of certain growth factors, such as EGF, bFGF, LIF and IGF-1.
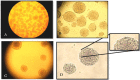
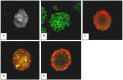
Methods: The NSCs were harvested and cultured from striatal tissue of 18 days old rat embryos. We have generated neurospheres from these NSCs and cultured them till passage 7 (28 days in vitro) under four different conditional microenvironments: (A) without growth factor, (B) EGF/bFGF, (C) EGF/bFGF/LIF, (D) EGF/bFGF/IGF-1 and (E) EGF/bFGF/LIF/IGF-1. Isolated NSCs were characterised by Immunoflouroscence for nestin expression. The cell growth and proliferation was evaluated at different time intervals (P1, P3, P5 & P7), assessing the metabolic activity based cell proliferation. Apoptosis was studied in each of these groups by In situ cell death assay.
Results: Our results demonstrated certain important findings relevant to long-term culture and maintenance of striatal NSC-derived neurospheres. This suggested that IGF-1 can induce enhanced cell proliferation during early stages of neurogenesis, impose long-term maintenance (up to passage 7) to cultured NSCs and enhance survival efficiency in vitro, in the presence of EGF and FGF.
Conclusions: Our findings support the hypothesis that the enforcement of IGF-1 treatment to the EGF/FGF-responsive NSCs, can lead to enhanced cell proliferation during early stages of neurogenesis, and an extended life span in vitro. This information will be beneficial for improving future therapeutic implication of NSCs, by addressing improved in vitro production of NSCs.
Keywords: EGF; IGF-1.; LIF; bFGF; neural stem cells; neurospheres; proliferative activity.
Conflict of interest statement
Competing interests: The authors declare that no competing interests exist.
Figures



Similar articles
-
IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells.BMC Neurosci. 2014 Jul 22;15:91. doi: 10.1186/1471-2202-15-91. BMC Neurosci. 2014. PMID: 25047045 Free PMC article.
-
Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2.J Neurosci. 2001 Sep 15;21(18):7194-202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. J Neurosci. 2001. PMID: 11549730 Free PMC article.
-
In vitro investigation of growth factors including MGF and IGF-1 in neural stem cell activation, proliferation, and migration.Brain Res. 2021 May 15;1759:147366. doi: 10.1016/j.brainres.2021.147366. Epub 2021 Feb 16. Brain Res. 2021. PMID: 33607046
-
Neural stem cell survival factors.Arch Biochem Biophys. 2013 Jun;534(1-2):71-87. doi: 10.1016/j.abb.2013.02.004. Epub 2013 Mar 5. Arch Biochem Biophys. 2013. PMID: 23470250 Review.
-
[Establishment and Maintenance of Adult Neural Stem Cells].Brain Nerve. 2017 Sep;69(9):1027-1034. doi: 10.11477/mf.1416200862. Brain Nerve. 2017. PMID: 28900065 Review. Japanese.
Cited by
-
CXCR7 Participates in CXCL12-mediated Cell Cycle and Proliferation Regulation in Mouse Neural Progenitor Cells.Curr Mol Med. 2016;16(8):738-746. doi: 10.2174/1566524016666160829153453. Curr Mol Med. 2016. PMID: 27573194 Free PMC article.
-
Growth factors-based beneficial effects of platelet lysate on umbilical cord-derived stem cells and their synergistic use in osteoarthritis treatment.Cell Death Dis. 2020 Oct 14;11(10):857. doi: 10.1038/s41419-020-03045-0. Cell Death Dis. 2020. PMID: 33057008 Free PMC article.
-
Activation of IGF-1/GLP-1 Signalling via 4-Hydroxyisoleucine Prevents Motor Neuron Impairments in Experimental ALS-Rats Exposed to Methylmercury-Induced Neurotoxicity.Molecules. 2022 Jun 16;27(12):3878. doi: 10.3390/molecules27123878. Molecules. 2022. PMID: 35745001 Free PMC article.
-
IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells.BMC Neurosci. 2014 Jul 22;15:91. doi: 10.1186/1471-2202-15-91. BMC Neurosci. 2014. PMID: 25047045 Free PMC article.
-
MicroRNA profiling reveals unique miRNA signatures in IGF-1 treated embryonic striatal stem cell fate decisions in striatal neurogenesis in vitro.Biomed Res Int. 2014;2014:503162. doi: 10.1155/2014/503162. Epub 2014 Sep 1. Biomed Res Int. 2014. PMID: 25254208 Free PMC article.
References
-
- Altman J, Bayer SA. Atlas of prenatal rat brain development. USA: CRC Press Ltd, London; 1995.
-
- Bazan E, Alonso FJM, Redondo C, Lopez MAT, Alfaro JM, Reimers D, Herranz AS, Paino CL, Serrano AB, Cobacho N, Caso E, Lobo MVT. et al. In vitro and in vivo characterization of neural stem cells. Histology and Histopathology: Cell Mol Biol. 2004;19(4):1261–1275. - PubMed
-
- Bez A, Corsini E, Curti D, Biggiogera M, Colombo A, Nicosia RF, Pagano SF, Parati EA. et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993(1-2):18–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

