Relative resistance to Mammalian target of rapamycin inhibition in vascular smooth muscle cells of diabetic donors
- PMID: 23532775
- PMCID: PMC3603189
Relative resistance to Mammalian target of rapamycin inhibition in vascular smooth muscle cells of diabetic donors
Abstract
Background: Diabetes mellitus is associated with an increased risk of cardiovascular disease. Intimal thickening, a component of cardiovascular disease, entails the proliferation and migration of vascular smooth muscle cells (VSMCs). Inhibition of the mammalian target of rapamycin (mTOR) blocks VSMC proliferation, in part through an increase in the cyclin-dependent kinase inhibitor, p27(Kip1). The use of mTOR inhibitors, such as rapamycin, is effective clinically in inhibiting intimal thickening. This efficacy is reduced in diabetic subjects, however, suggesting a change in the role of the mTOR pathway in intimal thickening under diabetic conditions.
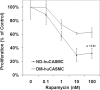
Methods: To examine whether diabetes induced changes in the role of mTOR in VSMC proliferation, we compared the response to rapamycin of human coronary artery VSMCs from diabetic (DM-huCASMC [human coronary artery smooth muscle cell]) and nondiabetic (ND-huCASMC) subjects.
Results: The DM-huCASMCs exhibited a relative resistance to rapamycin's inhibition of proliferation. Activation of the mTOR effector p70(S6kinase) was inhibited in rapamycin-treated DM-huCASMCs as in ND-huCASMCs. While ND-huCASMCs exhibited the normal increase in p27(Kip1) in response to rapamycin treatment, the DM-huCASMCs did not. Additionally, activation of the extracellular signal response kinase pathway was increased in the DM-huCASMCs, suggesting a potential pathway mediating the mTOR-independent decrease in p27(Kip1).
Conclusion: We conclude that diabetes is accompanied by a relative resistance to the effects of mTOR inhibition on VSMC proliferation through a loss of mTOR's effects on p27(Kip1) levels. These data provide insight into the effects of insulin resistance on the role of mTOR in regulating intimal thickening.
Keywords: Coronary artery disease; TOR serine-threonine kinases; cyclin-dependent kinase inhibitor p27; vascular smooth muscle.
Conflict of interest statement
Figures


Similar articles
-
Upregulation of miR-221 and -222 in response to increased extracellular signal-regulated kinases 1/2 activity exacerbates neointimal hyperplasia in diabetes mellitus.Atherosclerosis. 2018 Feb;269:71-78. doi: 10.1016/j.atherosclerosis.2017.12.016. Epub 2017 Dec 9. Atherosclerosis. 2018. PMID: 29276985 Free PMC article.
-
Loss of canonical insulin signaling accelerates vascular smooth muscle cell proliferation and migration through changes in p27Kip1 regulation.Endocrinology. 2011 Feb;152(2):651-8. doi: 10.1210/en.2010-0722. Epub 2010 Dec 29. Endocrinology. 2011. PMID: 21190963 Free PMC article.
-
The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation.Am J Physiol Cell Physiol. 2004 Mar;286(3):C507-17. doi: 10.1152/ajpcell.00201.2003. Epub 2003 Oct 30. Am J Physiol Cell Physiol. 2004. PMID: 14592809
-
mTOR Signaling Pathway in Bone Diseases Associated with Hyperglycemia.Int J Mol Sci. 2023 May 24;24(11):9198. doi: 10.3390/ijms24119198. Int J Mol Sci. 2023. PMID: 37298150 Free PMC article. Review.
-
mTOR Dysregulation, Insulin Resistance, and Hypertension.Biomedicines. 2024 Aug 8;12(8):1802. doi: 10.3390/biomedicines12081802. Biomedicines. 2024. PMID: 39200267 Free PMC article. Review.
Cited by
-
Dysregulation of the Mammalian Target of Rapamycin and p27Kip1 Promotes Intimal Hyperplasia in Diabetes Mellitus.Pharmaceuticals (Basel). 2013 May 27;6(6):716-27. doi: 10.3390/ph6060716. Pharmaceuticals (Basel). 2013. PMID: 24276258 Free PMC article.
-
Patient-tailored Drug-eluting Stent Choice - A Solution for Patients with Diabetes: Proceedings of Two Satellite Symposia Held at EuroPCR in May 2015 in Paris.Interv Cardiol. 2015 Sep;10(3):158-161. doi: 10.15420/ICR.2015.10.03.158. Interv Cardiol. 2015. PMID: 29588695 Free PMC article.
-
PCI in Patients with Diabetes: Role of the Cre8 Drug-eluting Stent.Interv Cardiol. 2017 May;12(1):13-17. doi: 10.15420/icr.2016:28:2. Interv Cardiol. 2017. PMID: 29588724 Free PMC article.
-
Recent publications by ochsner authors.Ochsner J. 2013 Summer;13(2):278-83. Ochsner J. 2013. PMID: 23789020 Free PMC article. No abstract available.
-
Stent Thrombosis and Restenosis with Contemporary Drug-Eluting Stents: Predictors and Current Evidence.J Clin Med. 2023 Feb 3;12(3):1238. doi: 10.3390/jcm12031238. J Clin Med. 2023. PMID: 36769886 Free PMC article. Review.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):e2–e220. Epub 2011 Dec 15. Erratum in: Circulation. 2012 Jun 5;125(22):e1002. - PMC - PubMed
-
- Wendt T, Bucciarelli L, Qu W, et al. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002 May;4(3):228–237. - PubMed
-
- Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR. Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology. 2007 May;148(5):2435–2443. Epub 2007 Jan 25. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
