Specific removal of TACC3-ch-TOG-clathrin at metaphase deregulates kinetochore fiber tension
- PMID: 23532825
- PMCID: PMC3666260
- DOI: 10.1242/jcs.124834
Specific removal of TACC3-ch-TOG-clathrin at metaphase deregulates kinetochore fiber tension
Abstract
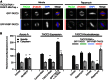
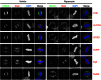
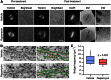
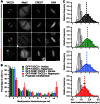
Microtubule-associated proteins of the mitotic spindle are thought to be important for the initial assembly and the maintenance of spindle structure and function. However, distinguishing assembly and maintenance roles for a given protein is difficult. Most experimental methods for protein inactivation are slow and therefore affect both assembly and maintenance. Here, we have used 'knocksideways' to rapidly (∼5 minutes) and specifically remove TACC3-ch-TOG-clathrin non-motor complexes from kinetochore fibers (K-fibers). This method allows the complex to be inactivated at defined stages of mitosis. Removal of TACC3-ch-TOG-clathrin after nuclear envelope breakdown caused severe delays in chromosome alignment. Inactivation at metaphase, following a normal prometaphase, significantly delayed progression to anaphase. In these cells, K-fiber tension was reduced and the spindle checkpoint was not satisfied. Surprisingly, there was no significant loss of K-fiber microtubules, even after prolonged removal. TACC3-ch-TOG-clathrin removal during metaphase also resulted in a decrease in spindle length and significant alteration in kinetochore dynamics. Our results indicate that TACC3-ch-TOG-clathrin complexes are important for the maintenance of spindle structure and function as well as for initial spindle assembly.
Keywords: Checkpoint; Knocksideways; Microtubule; Mitotic spindle; Rapid inactivation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

