Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members
- PMID: 23532839
- PMCID: PMC3650369
- DOI: 10.1074/jbc.M113.457937
Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members
Abstract
Background: TMEM16A and 16B work as Cl(-) channel, whereas 16F works as phospholipid scramblase. The function of other TMEM16 members is unknown.
Results: Using TMEM16F(-/-) cells, TMEM16C, 16D, 16F, 16G, and 16J were shown to be lipid scramblases.
Conclusion: Some TMEM16 members are divided into two Cl(-) channels and five lipid scramblases.
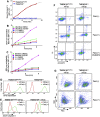
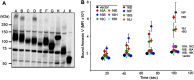
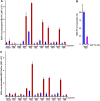
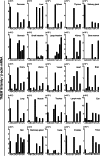
Significance: Learning the biochemical function ofTMEM16family members is essential to understand their physiological role. Asymmetrical distribution of phospholipids between the inner and outer plasma membrane leaflets is disrupted in various biological processes. We recently identified TMEM16F, an eight-transmembrane protein, as a Ca(2+)-dependent phospholipid scramblase that exposes phosphatidylserine (PS) to the cell surface. In this study, we established a mouse lymphocyte cell line with a floxed allele in the TMEM16F gene. When TMEM16F was deleted, these cells failed to expose PS in response to Ca(2+) ionophore, but PS exposure was elicited by Fas ligand treatment. We expressed other TMEM16 proteins in the TMEM16F(-/-) cells and found that not only TMEM16F, but also 16C, 16D, 16G, and 16J work as lipid scramblases with different preference to lipid substrates. On the other hand, a patch clamp analysis in 293T cells indicated that TMEM16A and 16B, but not other family members, acted as Ca(2+)-dependent Cl(-) channels. These results indicated that among 10 TMEM16 family members, 7 members could be divided into two subfamilies, Ca(2+)-dependent Cl(-) channels (16A and 16B) and Ca(2+)-dependent lipid scramblases (16C, 16D, 16F, 16G, and 16J).
Keywords: Calcium; Cl- Channel; Lipid Transport; Phosphatidylcholine; Phosphatidylserine; Phospholipid Scramblase; Plasma Membrane; TMEM16; Tissue-specific Expression.
Figures


References
-
- Balasubramanian K., Schroit A. (2003) Aminophospholipid asymmetry: a matter of life and death. Annu. Rev. Physiol. 65, 701–734 - PubMed
-
- Nagata S., Hanayama R., Kawane K. (2010) Autoimmunity and the clearance of dead cells. Cell 140, 619–630 - PubMed
-
- Zwaal R. F., Comfurius P., Bevers E. M. (1998) Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta 1376, 433–453 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

