Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy
- PMID: 23532841
- PMCID: PMC3663539
- DOI: 10.1074/jbc.M113.457408
Lewy body-like α-synuclein aggregates resist degradation and impair macroautophagy
Abstract
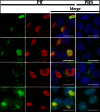
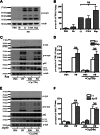
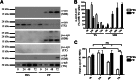
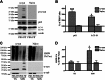
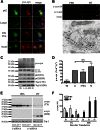
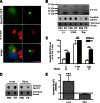
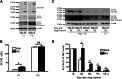
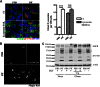
Cytoplasmic α-synuclein (α-syn) aggregates, referred to as Lewy bodies, are pathological hallmarks of a number of neurodegenerative diseases, most notably Parkinson disease. Activation of macroautophagy is suggested to facilitate degradation of certain proteinaceous inclusions, but it is unclear if this pathway is capable of degrading α-syn aggregates. Here, we examined this issue by utilizing cellular models in which intracellular Lewy body-like α-syn inclusions accumulate after internalization of pre-formed α-syn fibrils into α-syn-expressing HEK293 cells or cultured primary neurons. We demonstrate that α-syn inclusions cannot be effectively degraded, even though they co-localize with essential components of both the autophagic and proteasomal protein degradation pathways. The α-syn aggregates persist even after soluble α-syn levels have been substantially reduced, suggesting that once formed, the α-syn inclusions are refractory to clearance. Importantly, we also find that α-syn aggregates impair overall macroautophagy by reducing autophagosome clearance, which may contribute to the increased cell death that is observed in aggregate-bearing cells.
Keywords: Aggregation; Autophagy; Parkinson Disease; Protein Degradation; α-Synuclein.
Figures


References
-
- Spillantini M. G. (1999) Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy are α-synucleinopathies. Parkinsonism Relat. Disord. 5, 157–162 - PubMed
-
- Galvin J. E., Lee V. M., Trojanowski J. Q. (2001) Synucleinopathies: clinical and pathological implications. Arch. Neurol. 58, 186–190 - PubMed
-
- Kuzuhara S., Mori H., Izumiyama N., Yoshimura M., Ihara Y. (1988) Lewy bodies are ubiquitinated. A light and electron microscopic immunocytochemical study. Acta Neuropathol. 75, 345–353 - PubMed
-
- Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., Iwatsubo T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 - PubMed
-
- Wakabayashi K., Tanji K., Mori F., Takahashi H. (2007) The Lewy body in Parkinson's disease: molecules implicated in the formation and degradation of α-synuclein aggregates. Neuropathology 27, 494–506 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

