Structural and functional aspects of hetero-oligomers formed by the small heat shock proteins αB-crystallin and HSP27
- PMID: 23532854
- PMCID: PMC3650395
- DOI: 10.1074/jbc.M112.443812
Structural and functional aspects of hetero-oligomers formed by the small heat shock proteins αB-crystallin and HSP27
Abstract
Background: αB-crystallin and HSP27 are mammalian intracellular small heat shock proteins.
Results: These proteins exchange subunits in a rapid and temperature-dependent manner.
Conclusion: This facile subunit exchange suggests that differential expression could be used by the cell to regulate the response to stress.
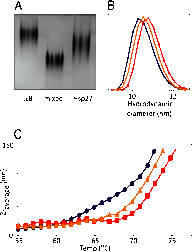
Significance: A robust technique defines parameters for the dynamic interaction between the major mammalian small heat shock proteins. Small heat shock proteins (sHSPs) exist as large polydisperse species in which there is constant dynamic subunit exchange between oligomeric and dissociated forms. Their primary role in vivo is to bind destabilized proteins and prevent their misfolding and aggregation. αB-Crystallin (αB) and HSP27 are the two most widely distributed and most studied sHSPs in the human body. They are coexpressed in different tissues, where they are known to associate with each other to form hetero-oligomeric complexes. In this study, we aimed to determine how these two sHSPs interact to form hetero-oligomers in vitro and whether, by doing so, there is an increase in their chaperone activity and stability compared with their homo-oligomeric forms. Our results demonstrate that HSP27 and αB formed polydisperse hetero-oligomers in vitro, which had an average molecular mass that was intermediate of each of the homo-oligomers and which were more thermostable than αB, but less so than HSP27. The hetero-oligomer chaperone function was found to be equivalent to that of αB, with each being significantly better in preventing the amorphous aggregation of α-lactalbumin and the amyloid fibril formation of α-synuclein in comparison with HSP27. Using mass spectrometry to monitor subunit exchange over time, we found that HSP27 and αB exchanged subunits 23% faster than the reported rate for HSP27 and αA and almost twice that for αA and αB. This represents the first quantitative evaluation of αB/HSP27 subunit exchange, and the results are discussed in the broader context of regulation of function and cellular proteostasis.
Keywords: Analytical Chemistry; Chaperone Chaperonin; Crystallin; HSP27; Mass Spectrometry (MS); Protein Aggregation; Small Heat Shock Proteins.
Figures

References
-
- Dobson C. M. (2003) Protein folding and misfolding. Nature 426, 884–890 - PubMed
-
- Chiti F., Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 - PubMed
-
- Muchowski P. J. (2002) Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 35, 9–12 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

