Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin
- PMID: 23532958
- PMCID: PMC3726575
- DOI: 10.1002/hep.26419
Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin
Abstract
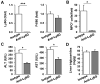
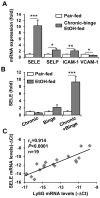
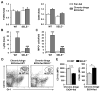
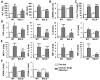
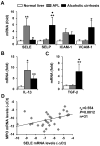
Chronic plus binge ethanol feeding acts synergistically to induce liver injury in mice, but the mechanisms underlying this phenomenon remain unclear. Here, we show that chronic plus binge ethanol feeding synergistically up-regulated the hepatic expression of interleukin-1β and tumor necrosis factor alpha and induced neutrophil accumulation in the liver, compared with chronic or binge feeding alone. In vivo depletion of neutrophils through administration of an anti-Ly6G antibody markedly reduced chronic-binge ethanol feeding-induced liver injury. Real-time polymerase chain reaction analyses revealed that hepatic E-selectin expression was up-regulated 10-fold, whereas expression of other neutrophil infiltration-related adhesion molecules (e.g., P-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1) was slightly up- or down-regulated in this chronic-binge model. The genetic deletion of E-selectin prevented chronic-binge ethanol-induced hepatic neutrophil infiltration as well as elevation of serum transaminases without affecting ethanol-induced steatosis. In addition, E-selectin-deficient mice showed reduced hepatic expression of several proinflammatory cytokines, chemokines, and adhesion molecules, compared to wild-type mice, after chronic-binge ethanol feeding. Finally, the expression of E-selectin was highly up-regulated in human alcoholic fatty livers, but not in alcoholic cirrhosis.
Conclusions: Chronic-binge ethanol feeding up-regulates expression of proinflammatory cytokines, followed by the induction of E-selectin. Elevated E-selectin plays an important role in hepatic neutrophil infiltration and injury induced by chronic-binge feeding in mice and may also contribute to the pathogenesis of early stages of human alcoholic liver disease.
© 2013 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
No conflicts of interest exist for any of the authors.
Figures



Comment in
-
Build a better mouse model, and the world will beat a path to your door.Hepatology. 2013 Nov;58(5):1526-8. doi: 10.1002/hep.26596. Epub 2013 Sep 13. Hepatology. 2013. PMID: 23813457 Free PMC article. No abstract available.
References
-
- O’Shea RS, Dasarathy S, McCullough AJ Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–28. - PubMed
-
- Anstee QM, Daly AK, Day CP. Genetics of alcoholic and nonalcoholic fatty liver disease. Semin Liver Dis. 2011;31:128–46. - PubMed
-
- Li TK. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol. 2008;23 (Suppl 1):S2–8. - PubMed
-
- Hatton J, Burton A, Nash H, Munn E, Burgoyne L, Sheron N. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction. 2009;104:587–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
