Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators
- PMID: 23532979
- PMCID: PMC3622923
- DOI: 10.1128/mBio.00636-12
Inhibition of viral pathogenesis and promotion of the septic shock response to bacterial infection by IRF-3 are regulated by the acetylation and phosphorylation of its coactivators
Abstract
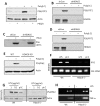
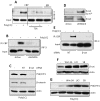
Interferon (IFN) is required for protecting mice from viral pathogenesis; reciprocally, it mediates the deleterious septic shock response to bacterial infection. The critical transcription factor for IFN induction, in both cases, is IRF-3, which is activated by TLR3 or RIG-I signaling in response to virus infection and TLR4 signaling in response to bacterial infection. Here, we report that IRF-3's transcriptional activity required its coactivators, β-catenin and CBP, to be modified by HDAC6-mediated deacetylation and protein kinase C isozyme β (PKC-β)-mediated phosphorylation, respectively, so that activated nuclear IRF-3 could form a stable transcription initiation complex at the target gene promoters. β-Catenin bridges IRF-3 and CBP, and the modifications were required specifically for the interaction between β-catenin and CBP but not β-catenin and IRF-3. Consequently, like IRF-3(-/-) mice, HDAC6(-/-) mice were resistant to bacterial lipopolysaccharide-induced septic shock. Conversely, they were highly susceptible to pathogenesis caused by Sendai virus infection. Thus, HDAC6 is an essential component of the innate immune response to microbial infection.
Figures



References
-
- Fensterl V, Sen GC. 2009. Interferons and viral infections. Biofactors 35:14–20 - PubMed
-
- Karaghiosoff M, Steinborn R, Kovarik P, Kriegshäuser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Müller M. 2003. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat. Immunol. 4:471–477 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

