Adherence to medication is a more important contributor to viral breakthrough in chronic hepatitis B patients treated with entecavir than in those with Lamivudine
- PMID: 23533048
- PMCID: PMC3607242
- DOI: 10.7150/ijms.5795
Adherence to medication is a more important contributor to viral breakthrough in chronic hepatitis B patients treated with entecavir than in those with Lamivudine
Abstract
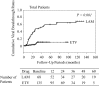
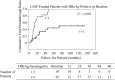
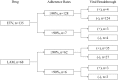
Viral breakthrough is related to poor adherence to medication in some chronic hepatitis B patients treated with nucleos(t)ide analogues (NAs). Our study aimed to examine how adherence to medication is associated with viral breakthrough in patients treated with NAs. A total of 203 patients (135 ETV and 68 LAM) were analyzed in this retrospective analysis. Physical examination, serum liver enzyme tests, and hepatitis B virus marker tests were performed at least every 3 months. We reviewed medical records and performed medical interviews regarding to patients' adherence to medication. Adherence rates <90% were defined as poor adherence in the present study. Cumulative viral breakthrough rates were lower in the ETV-treated patients than in the LAM-treated patients (P<0.001). Seven ETV-treated (5.1%) and 6 LAM-treated patients (8.8%) revealed poor adherence to medication (P=0.48). Among ETV-treated patients, 4 (3.1%) of 128 patients without poor adherence experienced viral breakthrough and 3 (42.8%) of 7 patients with poor adherence experienced viral breakthrough (P<0.001). Only 3 of 38 (7.8%) LAM-treated patients with viral breakthrough had poor adherence, a lower rate than the ETV-treated patients (P=0.039). Nucleoside analogue resistance mutations were observed in 50.0% of ETV- and 94.1% of LAM-treated patients with viral breakthrough (P=0.047). Viral breakthrough associated with poor adherence could be a more important issue in the treatment with especially stronger NAs, such as ETV.
Keywords: Adherence; Entecavir; Hepatitis B; Lamivudine; Viral Breakthrough..
Conflict of interest statement
COMPETING INTERESTS: Dr. Tatsuo Kanda reports receiving lecture fees from Chugai Pharmaceutical, MSD, and Ajinomoto, and Prof. Osamu Yokosuka reports receiving grant support from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, and Bristol-Myers Squibb.
Figures

References
-
- Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. - PubMed
-
- Wu S, Fukai K, Imazeki F. et al. Initial virological response and viral mutation with adefovir dipivoxil added to ongoing Lamivudine therapy in Lamivudine-resistant chronic hepatitis B. Dig Dis Sci. 2011;56:1207–1214. - PubMed
-
- Dienstag JL, Perrillo RP, Schiff ER. et al. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. - PubMed
-
- Lai CL, Chien RN, Leung NW. et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

