Type I interferon receptor deficiency prevents murine Sjogren's syndrome
- PMID: 23533183
- PMCID: PMC3627507
- DOI: 10.1177/0022034513483315
Type I interferon receptor deficiency prevents murine Sjogren's syndrome
Abstract
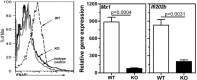
In Sjögren's Syndrome (SS), inherent glandular defects, autoimmunity, and mononuclear cell infiltration within the salivary glands cause reduced salivation leading to xerostomia. Excessive production of type I interferons (IFN), triggered by environmental and genetic factors, is considered pathogenic in this disorder. However, whether type I IFN production is causative or an outcome of the disease process is not known. To address this question, we introduced a deficiency of interferon alpha receptor 1 (Ifnar1) into B6.Aec1Aec2 mice, which are known to have the genetic loci necessary for developing a SS-like disorder. This new mouse strain, B6.Aec1Aec2Ifnar1 (-/-), lacking type I IFN-mediated signaling, was characterized for pilocarpine-induced salivation, the presence of serum autoantibodies, sialoadenitis, and dacryoadenitis. Compared with the B6.Aec1Aec2Ifnar1 (+/+) (wild-type) mice, the B6.Aec1Aec2Ifnar1 (-/-) (knockout) mice had significantly lower mononuclear cell infiltration in the salivary and lacrimal glands. The knockout mice were completely protected from salivary gland dysfunction. Surprisingly, they had a robust autoantibody response comparable with that of the wild-type mice. These findings demonstrate that, in the absence of type I IFN-mediated signaling, systemic autoantibody responses can be dissociated from glandular pathology. Our study suggests that, in genetically susceptible individuals, the type I IFN pathway can instigate certain features of SS.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Bagavant H, Thompson C, Ohno K, Setiady Y, Tung KS. (2002). Differential effect of neonatal thymectomy on systemic and organ-specific autoimmune disease. Int Immunol 14:1397-1406. - PubMed
-
- Carr AJ, Ng WF, Figueiredo F, Macleod RI, Greenwood M, Staines K. (2012). Sjögren’s syndrome— an update for dental practitioners. Br Dent J 213:353-357. - PubMed
-
- Cha S, Nagashima H, Brown VB, Peck AB, Humphreys-Beher MG. (2002). Two NOD Idd associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis Rheum 46:1390-1398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

