Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas
- PMID: 23533272
- PMCID: PMC3625308
- DOI: 10.1073/pnas.1219232110
Paradoxical activation and RAF inhibitor resistance of BRAF protein kinase fusions characterizing pediatric astrocytomas
Erratum in
- Proc Natl Acad Sci U S A. 2013 May 21;110(21):8750
Abstract
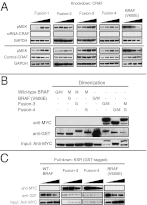
Astrocytomas are the most common type of brain tumors in children. Activated BRAF protein kinase mutations are characteristic of pediatric astrocytomas with KIAA1549-BRAF fusion genes typifying low-grade astrocytomas and (V600E)BRAF alterations characterizing distinct or higher-grade tumors. Recently, BRAF-targeted therapies, such as vemurafenib, have shown great promise in treating V600E-dependent melanomas. Like (V600E)BRAF, BRAF fusion kinases activate MAPK signaling and are sufficient for malignant transformation; however, here we characterized the distinct mechanisms of action of KIAA1549-BRAF and its differential responsiveness to PLX4720, a first-generation BRAF inhibitor and research analog of vemurafenib. We found that in cells expressing KIAA1549-BRAF, the fusion kinase functions as a homodimer that is resistant to PLX4720 and accordingly is associated with CRAF-independent paradoxical activation of MAPK signaling. Mutagenesis studies demonstrated that KIAA1549-BRAF fusion-mediated signaling is diminished with disruption of the BRAF kinase dimer interface. In addition, the KIAA1549-BRAF fusion displays increased binding affinity to kinase suppressor of RAS (KSR), an RAF relative recently demonstrated to facilitate MEK phosphorylation by BRAF. Despite its resistance to PLX4720, the KIAA1549-BRAF fusion is responsive to a second-generation selective BRAF inhibitor that, unlike vemurafenib, does not induce activation of wild-type BRAF. Our data support the development of targeted treatment paradigms for BRAF-altered pediatric astrocytomas and also demonstrate that therapies must be tailored to the specific mutational context and distinct mechanisms of action of the mutant kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Montagut C, Settleman J. Targeting the RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 2009;283(2):125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

