Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism
- PMID: 23533304
- PMCID: PMC3596927
- DOI: 10.1155/2013/328797
Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism
Abstract
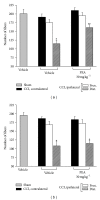
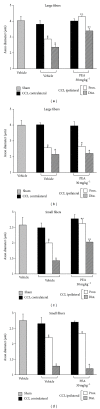
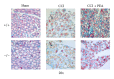
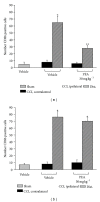
Neuropathic syndromes which are evoked by lesions to the peripheral or central nervous system are extremely difficult to treat, and available drugs rarely joint an antihyperalgesic with a neurorestorative effect. N-Palmitoylethanolamine (PEA) exerts antinociceptive effects in several animal models and inhibits peripheral inflammation in rodents. Aimed to evaluate the antineuropathic properties of PEA, a damage of the sciatic nerve was induced in mice by chronic constriction injury (CCI) and a subcutaneous daily treatment with 30 mg kg(-1) PEA was performed. On the day 14, PEA prevented pain threshold alterations. Histological studies highlighted that CCI induced oedema and an important infiltrate of CD86 positive cells in the sciatic nerve. Moreover, osmicated preparations revealed a decrease in axon diameter and myelin thickness. Repeated treatments with PEA reduced the presence of oedema and macrophage infiltrate, and a significant higher myelin sheath, axonal diameter, and a number of fibers were observable. In PPAR- α null mice PEA treatment failed to induce pain relief as well as to rescue the peripheral nerve from inflammation and structural derangement. These results strongly suggest that PEA, via a PPAR- α -mediated mechanism, can directly intervene in the nervous tissue alterations responsible for pain, starting to prevent macrophage infiltration.
Figures






Similar articles
-
The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB(1), TRPV1 and PPARgamma receptors and neurotrophic factors.Pain. 2008 Oct 31;139(3):541-550. doi: 10.1016/j.pain.2008.06.003. Epub 2008 Jul 3. Pain. 2008. PMID: 18602217
-
Molecular evidence for the involvement of PPAR-δ and PPAR-γ in anti-inflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma.J Neuroinflammation. 2013 Feb 1;10:20. doi: 10.1186/1742-2094-10-20. J Neuroinflammation. 2013. PMID: 23374874 Free PMC article.
-
Efficacy of a fixed combination of palmitoylethanolamide and acetyl-l-carnitine (PEA+ALC FC) in the treatment of neuropathies secondary to rheumatic diseases.Minerva Med. 2021 Aug;112(4):492-499. doi: 10.23736/S0026-4806.21.07486-3. Epub 2021 May 31. Minerva Med. 2021. PMID: 34056884
-
Mechanisms and clinical applications of palmitoylethanolamide (PEA) in the treatment of neuropathic pain.Inflammopharmacology. 2025 Jan;33(1):121-133. doi: 10.1007/s10787-024-01623-8. Epub 2024 Dec 23. Inflammopharmacology. 2025. PMID: 39714723 Review.
-
Palmitoylethanolamide is a new possible pharmacological treatment for the inflammation associated with trauma.Mini Rev Med Chem. 2013 Feb;13(2):237-55. Mini Rev Med Chem. 2013. PMID: 22697514 Review.
Cited by
-
Pharmacological Blockade of PPARα Exacerbates Inflammatory Pain-Related Impairment of Spatial Memory in Rats.Biomedicines. 2021 May 27;9(6):610. doi: 10.3390/biomedicines9060610. Biomedicines. 2021. PMID: 34072060 Free PMC article.
-
Effects of the glial modulator palmitoylethanolamide on chronic pain intensity and brain function.J Pain Res. 2019 Aug 2;12:2427-2439. doi: 10.2147/JPR.S209657. eCollection 2019. J Pain Res. 2019. PMID: 31447580 Free PMC article.
-
Micronized Palmitoylethanolamide: A Post Hoc Analysis of a Controlled Study in Patients with Low Back Pain - Sciatica.CNS Neurol Disord Drug Targets. 2019;18(6):491-495. doi: 10.2174/1871527318666190703110036. CNS Neurol Disord Drug Targets. 2019. PMID: 31269891 Free PMC article. Clinical Trial.
-
PPARs and pain.Br J Pharmacol. 2019 May;176(10):1421-1442. doi: 10.1111/bph.14339. Epub 2018 Jun 3. Br J Pharmacol. 2019. PMID: 29679493 Free PMC article. Review.
-
Antineuropathic profile of N-palmitoylethanolamine in a rat model of oxaliplatin-induced neurotoxicity.PLoS One. 2015 Jun 3;10(6):e0128080. doi: 10.1371/journal.pone.0128080. eCollection 2015. PLoS One. 2015. PMID: 26039098 Free PMC article.
References
-
- Dyck PJ. The causes, classification and treatment of peripheral neuropathy. The New England Journal of Medicine. 1982;307:283–286. - PubMed
-
- Baron R. Mechanisms of disease: neuropathic pain—a clinical perspective. Nature Clinical Practice Neurology. 2006;2:95–106. - PubMed
-
- Tal M. A role for inflammation in chronic pain. Current Review of Pain. 1999;3:440–446. - PubMed
-
- Vivoli E, Di Cesare Mannelli L, Salvicchi A, et al. Acetyl-l-carnitine increases artemin level and prevents neurotrophic factor alterations during neuropathy. Neuroscience. 2010;167(4):1168–1174. - PubMed
-
- Üçeyler N, Göbel K, Meuth SG, et al. Deficiency of the negative immune regulator B7-H1 enhances inflammation and neuropathic pain after chronic constriction injury of mouse sciatic nerve. Experimental Neurology. 2010;222(1):153–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

