Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis
- PMID: 23533410
- PMCID: PMC3603547
- DOI: 10.1155/2013/897025
Mammalian target of rapamycin inhibitors induce tumor cell apoptosis in vivo primarily by inhibiting VEGF expression and angiogenesis
Abstract
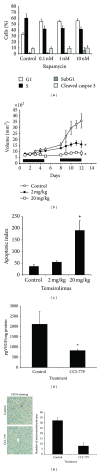
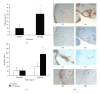
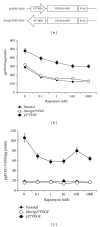
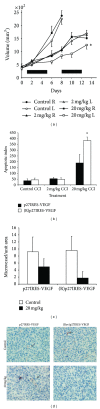
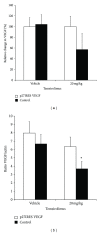
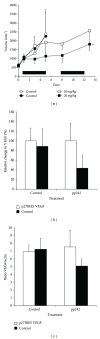
We found that rapalog mTOR inhibitors induce G1 arrest in the PTEN-null HS Sultan B-cell lymphoma line in vitro, but that administration of rapalogs in a HS Sultan xenograft model resulted in significant apoptosis, and that this correlated with induction of hypoxia and inhibition of neoangiogenesis and VEGF expression. Mechanistically, rapalogs prevent cap-dependent translation, but studies have shown that cap-independent, internal ribosome entry site (IRES)-mediated translation of genes, such as c-myc and cyclin D, can provide a fail-safe mechanism that regulates tumor survival. Therefore, we tested if IRES-dependent expression of VEGF could likewise regulate sensitivity of tumor cells in vivo. To achieve this, we developed isogenic HS Sultan cell lines that ectopically express the VEGF ORF fused to the p27 IRES, an IRES sequence that is insensitive to AKT-mediated inhibition of IRES activity and effective in PTEN-null tumors. Mice challenged with p27-VEGF transfected tumor cells were more resistant to the antiangiogenic and apoptotic effects of the rapalog, temsirolimus, and active site mTOR inhibitor, pp242. Our results confirm the critical role of VEGF expression in tumors during treatment with mTOR inhibitors and underscore the importance of IRES activity as a resistance mechanism to such targeted therapy.
Figures
Similar articles
-
AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells.Oncogene. 2007 Apr 5;26(16):2255-62. doi: 10.1038/sj.onc.1210019. Epub 2006 Oct 2. Oncogene. 2007. PMID: 17016437
-
Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway.J Biol Chem. 2005 Mar 25;280(12):10964-73. doi: 10.1074/jbc.M407874200. Epub 2005 Jan 4. J Biol Chem. 2005. PMID: 15634685
-
Mechanistic Target of Rapamycin (mTOR) Inhibition Synergizes with Reduced Internal Ribosome Entry Site (IRES)-mediated Translation of Cyclin D1 and c-MYC mRNAs to Treat Glioblastoma.J Biol Chem. 2016 Jul 1;291(27):14146-14159. doi: 10.1074/jbc.M116.726927. Epub 2016 May 11. J Biol Chem. 2016. PMID: 27226604 Free PMC article.
-
Mammalian target of rapamycin inhibition as therapy for hematologic malignancies.Cancer. 2004 Feb 15;100(4):657-66. doi: 10.1002/cncr.20026. Cancer. 2004. PMID: 14770419 Review.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
Cited by
-
Tumour growth of colorectal rat liver metastases is inhibited by hepatic arterial infusion of the mTOR-inhibitor temsirolimus after portal branch ligation.Clin Exp Metastasis. 2015 Apr;32(4):313-21. doi: 10.1007/s10585-015-9707-4. Epub 2015 Feb 19. Clin Exp Metastasis. 2015. PMID: 25693517
-
The hypoxia signalling pathway in haematological malignancies.Oncotarget. 2017 May 30;8(22):36832-36844. doi: 10.18632/oncotarget.15981. Oncotarget. 2017. PMID: 28415662 Free PMC article. Review.
-
Efficacy and safety of a mammalian target of rapamycin inhibitor in pediatric patients with tuberous sclerosis complex: A systematic review and meta-analysis.Exp Ther Med. 2015 Feb;9(2):626-630. doi: 10.3892/etm.2014.2093. Epub 2014 Nov 27. Exp Ther Med. 2015. PMID: 25574245 Free PMC article.
-
Restoration of the prolyl-hydroxylase domain protein-3 oxygen-sensing mechanism is responsible for regulation of HIF2α expression and induction of sensitivity of myeloma cells to hypoxia-mediated apoptosis.PLoS One. 2017 Dec 5;12(12):e0188438. doi: 10.1371/journal.pone.0188438. eCollection 2017. PLoS One. 2017. PMID: 29206844 Free PMC article.
-
Recent advances of nanotechnology-based tumor vessel-targeting strategies.J Nanobiotechnology. 2021 Dec 20;19(1):435. doi: 10.1186/s12951-021-01190-y. J Nanobiotechnology. 2021. PMID: 34930293 Free PMC article. Review.
References
-
- Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. Journal of Clinical Oncology. 2004;22(5):909–918. - PubMed
-
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. The New England Journal of Medicine. 2007;356:2271–2281. - PubMed
-
- Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104(13):4188–4193. - PubMed
-
- Zangari M, Cavallo F, Tricot G. Farnesyltransferase inhibitors and rapamycin in the treatment of multiple myeloma. Current Pharmaceutical Biotechnology. 2006;7(6):449–453. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

