Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling
- PMID: 23533608
- PMCID: PMC3606479
- DOI: 10.1371/journal.pone.0059252
Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/6a917a2e-df4a-4ad9-99bb-6aa7218b833e
Abstract
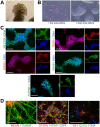
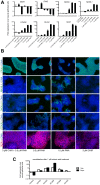
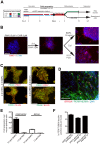
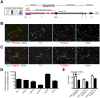
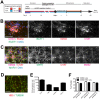
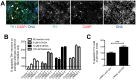
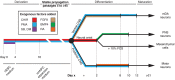
Phenotypic drug discovery requires billions of cells for high-throughput screening (HTS) campaigns. Because up to several million different small molecules will be tested in a single HTS campaign, even small variability within the cell populations for screening could easily invalidate an entire campaign. Neurodegenerative assays are particularly challenging because neurons are post-mitotic and cannot be expanded for implementation in HTS. Therefore, HTS for neuroprotective compounds requires a cell type that is robustly expandable and able to differentiate into all of the neuronal subtypes involved in disease pathogenesis. Here, we report the derivation and propagation using only small molecules of human neural progenitor cells (small molecule neural precursor cells; smNPCs). smNPCs are robust, exhibit immortal expansion, and do not require cumbersome manual culture and selection steps. We demonstrate that smNPCs have the potential to clonally and efficiently differentiate into neural tube lineages, including motor neurons (MNs) and midbrain dopaminergic neurons (mDANs) as well as neural crest lineages, including peripheral neurons and mesenchymal cells. These properties are so far only matched by pluripotent stem cells. Finally, to demonstrate the usefulness of smNPCs we show that mDANs differentiated from smNPCs with LRRK2 G2019S are more susceptible to apoptosis in the presence of oxidative stress compared to wild-type. Therefore, smNPCs are a powerful biological tool with properties that are optimal for large-scale disease modeling, phenotypic screening, and studies of early human development.
Conflict of interest statement
Figures

References
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

