HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes
- PMID: 23533630
- PMCID: PMC3606318
- DOI: 10.1371/journal.pone.0059514
HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes
Abstract
Background: HIV protease inhibitors (PI) are core components of Highly Active Antiretroviral Therapy (HAART), the most effective treatment for HIV infection currently available. However, HIV PIs have now been linked to lipodystrophy and dyslipidemia, which are major risk factors for cardiovascular disease and metabolic syndrome. Our previous studies have shown that HIV PIs activate endoplasmic reticulum (ER) stress and disrupt lipid metabolism in hepatocytes and macrophages. Yet, little is known on how HIV PIs disrupt lipid metabolism in adipocytes, a major cell type involved in the pathogenesis of metabolic syndrome.
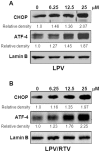
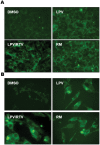
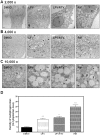
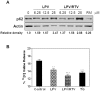
Methodology and principal findings: Cultured and primary mouse adipocytes and human adipocytes were used to examine the effect of frequently used HIV PIs in the clinic, lopinavir/ritonavir, on adipocyte differentiation and further identify the underlying molecular mechanism of HIV PI-induced dysregulation of lipid metabolism in adipocytes. The results indicated that lopinavir alone or in combination with ritonavir, significantly activated the ER stress response, inhibited cell differentiation, and induced cell apoptosis in adipocytes. In addition, HIV PI-induced ER stress was closely linked to inhibition of autophagy activity. We also identified through the use of primary adipocytes of CHOP(-/-) mice that CHOP, the major transcriptional factor of the ER stress signaling pathway, is involved in lopinavir/ritonavir-induced inhibition of cell differentiation in adipocytes. In addition, lopinavir/ritonavir-induced ER stress appears to be associated with inhibition of autophagy activity in adipocytes.
Conclusion and significance: Activation of ER stress and impairment of autophagy activity are involved in HIV PI-induced dysregulation of lipid metabolism in adipocytes. The key components of ER stress and autophagy signaling pathways are potential therapeutic targets for HIV PI-induced metabolic side effects in HIV patients.
Conflict of interest statement
Figures








Similar articles
-
The role of CCAAT enhancer-binding protein homologous protein in human immunodeficiency virus protease-inhibitor-induced hepatic lipotoxicity in mice.Hepatology. 2013 Mar;57(3):1005-16. doi: 10.1002/hep.26107. Epub 2013 Feb 12. Hepatology. 2013. PMID: 23080229 Free PMC article.
-
Prevention of HIV protease inhibitor-induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways.J Pharmacol Exp Ther. 2010 Aug;334(2):530-9. doi: 10.1124/jpet.110.168484. Epub 2010 May 14. J Pharmacol Exp Ther. 2010. PMID: 20472667 Free PMC article.
-
HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells.Gastroenterology. 2010 Jan;138(1):197-209. doi: 10.1053/j.gastro.2009.08.054. Epub 2009 Sep 2. Gastroenterology. 2010. PMID: 19732776 Free PMC article.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
Cited by
-
Cellular Mechanism Underlying Highly-Active or Antiretroviral Therapy-Induced Lipodystrophy: Atazanavir, a Protease Inhibitor, Compromises Adipogenic Conversion of Adipose-Derived Stem/Progenitor Cells through Accelerating ER Stress-Mediated Cell Death in Differentiating Adipocytes.Int J Mol Sci. 2021 Feb 20;22(4):2114. doi: 10.3390/ijms22042114. Int J Mol Sci. 2021. PMID: 33672735 Free PMC article.
-
Hypertension among HIV-infected adults receiving highly active antiretroviral therapy (HAART) in Malaysia.Glob J Health Sci. 2013 Dec 1;6(2):58-71. doi: 10.5539/gjhs.v6n2p58. Glob J Health Sci. 2013. PMID: 24576366 Free PMC article.
-
Lopinavir, an HIV-1 peptidase inhibitor, induces alteration on the lipid metabolism of Leishmania amazonensis promastigotes.Parasitology. 2018 Sep;145(10):1304-1310. doi: 10.1017/S0031182018000823. Epub 2018 May 28. Parasitology. 2018. PMID: 29806577 Free PMC article.
-
Alcoholic Extract of Lotus Leaves Improves Lipid Profile in Rats with HIV Protease Inhibitor-induced Dyslipidaemia.West Indian Med J. 2015 Jun;64(3):195-200. doi: 10.7727/wimj.2014.373. Epub 2015 Jul 21. West Indian Med J. 2015. PMID: 26426169 Free PMC article.
-
Gastrointestinal and hepatic diseases during the COVID-19 pandemic: Manifestations, mechanism and management.World J Gastroenterol. 2021 Jul 28;27(28):4504-4535. doi: 10.3748/wjg.v27.i28.4504. World J Gastroenterol. 2021. PMID: 34366621 Free PMC article. Review.
References
-
- Carr A, Cooper DA (1998) Images in clinical medicine. Lipodystrophy associated with an HIV-protease inhibitor. The New England journal of medicine 339: 1296. - PubMed
-
- Group DADS, Friis-Moller N, Reiss P, Sabin CA, Weber R, et al (2007) Class of antiretroviral drugs and the risk of myocardial infarction. The New England journal of medicine 356: 1723–1735. - PubMed
-
- Zhou H, Pandak WM Jr, Lyall V, Natarajan R, Hylemon PB (2005) HIV protease inhibitors activate the unfolded protein response in macrophages: implication for atherosclerosis and cardiovascular disease. Molecular pharmacology 68: 690–700. - PubMed
-
- Zhou H, Gurley EC, Jarujaron S, Ding H, Fang Y, et al. (2006) HIV protease inhibitors activate the unfolded protein response and disrupt lipid metabolism in primary hepatocytes. American journal of physiologyGastrointestinal and liver physiology 291: G1071–1080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

