Neutralizing antibody responses in macaques induced by human immunodeficiency virus type 1 monovalent or trivalent envelope glycoproteins
- PMID: 23533650
- PMCID: PMC3606129
- DOI: 10.1371/journal.pone.0059803
Neutralizing antibody responses in macaques induced by human immunodeficiency virus type 1 monovalent or trivalent envelope glycoproteins
Abstract
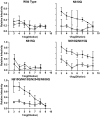
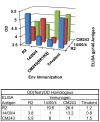
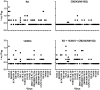
A major goal of efforts to develop a vaccine to prevent HIV-1 infection is induction of broadly cross-reactive neutralizing antibodies (bcnAb). In previous studies we have demonstrated induction of neutralizing antibodies that did cross-react among multiple primary and laboratory strains of HIV-1, but neutralized with limited potency. In the present study we tested the hypothesis that immunization with multiple HIV-1 envelope glycoproteins (Envs) would result in a more potent and cross-reactive neutralizing response. One Env, CM243(N610Q), was selected on the basis of studies of the effects of single and multiple mutations of the four gp41 glycosylation sites. The other two Envs included R2 (subtype B) and 14/00/4 (subtype F), both of which were obtained from donors with bcnAb. Rhesus monkeys were immunized using a prime boost regimen as in previous studies. Individual groups of monkeys were immunized with either one of the three Envs or all three. The single N610Q and N615Q mutations of CM243 Env did not disrupt protein secretion, processing into, or reactivity with mAbs, unlike other single or multiple deglycosylation mutations. In rabbit studies the N610Q mutation alone or in combination was associated with an enhanced neutralizing response against homologous and heterologous subtype E viruses. In the subsequent monkey study the response induced by the R2 Env regimen was equivalent to the trivalent regimen and superior to the other monovalent regimens against the virus panel used for testing. The 14/00/4 Env induced responses superior to CM243(N610Q). The results indicate that elimination of the glycosylation site near the gp41 loop results in enhanced immunogenicity, but that immunization of monkeys with these three distinct Envs was not more immunogenic than with one.
Conflict of interest statement
Figures


References
-
- Beirnaert E, De Zutter S, Janssens W, van der Groen G (2001) Potent broad cross-neutralizing sera inhibit attachment of primary HIV-1 isolates (groups M and O) to peripheral blood mononuclear cells. Virology 281: 305–314. - PubMed
-
- Quinnan GV Jr, Zhang PF, Fu DW, Dong M, Alter HJ (1999) Expression and characterization of HIV type 1 envelope protein associated with a broadly reactive neutralizing antibody response. AIDS Res Hum Retroviruses 15: 561–570. - PubMed
-
- Donners H, Willems B, Beirnaert E, Colebunders R, Davis D, et al. (2002) Cross-neutralizing antibodies against primary isolates in African women infected with HIV-1. Aids 16: 501–503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

