Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells
- PMID: 23533656
- PMCID: PMC3606290
- DOI: 10.1371/journal.pone.0059890
Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells
Abstract
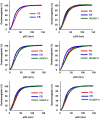
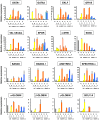
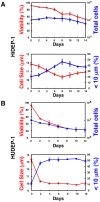
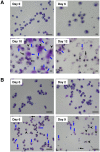
Transfusion of red blood cells (RBCs) is a standard and indispensable therapy in current clinical practice. In vitro production of RBCs offers a potential means to overcome a shortage of transfusable RBCs in some clinical situations and also to provide a source of cells free from possible infection or contamination by microorganisms. Thus, in vitro production of RBCs may become a standard procedure in the future. We previously reported the successful establishment of immortalized mouse erythroid progenitor cell lines that were able to produce mature RBCs very efficiently. Here, we have developed a reliable protocol for establishing immortalized human erythroid progenitor cell lines that are able to produce enucleated RBCs. These immortalized cell lines produce functional hemoglobin and express erythroid-specific markers, and these markers are upregulated following induction of differentiation in vitro. Most importantly, these immortalized cell lines all produce enucleated RBCs after induction of differentiation in vitro, although the efficiency of producing enucleated RBCs remains to be improved further. To the best of our knowledge, this is the first demonstration of the feasibility of using immortalized human erythroid progenitor cell lines as an ex vivo source for production of enucleated RBCs.
Conflict of interest statement
Figures
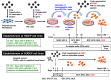
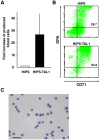
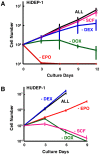
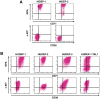

References
-
- Anstee DJ (2010) Production of erythroid cells from human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPS). Transfus Clin Biol 17: 104–109. - PubMed
-
- Douay L, Lapillonne H, Turhan AG (2009) Stem cells–a source of adult red blood cells for transfusion purposes: present and future. Crit Care Clin 25: 383–398, Table of Contents. - PubMed
-
- Migliaccio AR, Whitsett C, Migliaccio G (2009) Erythroid cells in vitro: from developmental biology to blood transfusion products. Curr Opin Hematol 16: 259–268. - PubMed
-
- Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y (2006) Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol 24: 1255–1256. - PubMed
-
- Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, et al. (2002) Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol 20: 467–472. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

