Melanoma-Targeted Chemothermotherapy and In Situ Peptide Immunotherapy through HSP Production by Using Melanogenesis Substrate, NPrCAP, and Magnetite Nanoparticles
- PMID: 23533767
- PMCID: PMC3595688
- DOI: 10.1155/2013/742925
Melanoma-Targeted Chemothermotherapy and In Situ Peptide Immunotherapy through HSP Production by Using Melanogenesis Substrate, NPrCAP, and Magnetite Nanoparticles
Abstract
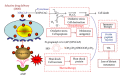
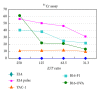
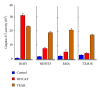
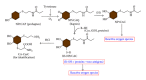
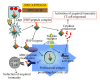
Exploitation of biological properties unique to cancer cells may provide a novel approach to overcome difficult challenges to the treatment of advanced melanoma. In order to develop melanoma-targeted chemothermoimmunotherapy, a melanogenesis substrate, N-propionyl-4-S-cysteaminylphenol (NPrCAP), sulfur-amine analogue of tyrosine, was conjugated with magnetite nanoparticles. NPrCAP was exploited from melanogenesis substrates, which are expected to be selectively incorporated into melanoma cells and produce highly reactive free radicals through reacting with tyrosinase, resulting in chemotherapeutic and immunotherapeutic effects by oxidative stress and apoptotic cell death. Magnetite nanoparticles were conjugated with NPrCAP to introduce thermotherapeutic and immunotherapeutic effects through nonapoptotic cell death and generation of heat shock protein (HSP) upon exposure to alternating magnetic field (AMF). During these therapeutic processes, NPrCAP was also expected to provide melanoma-targeted drug delivery system.
Figures




References
-
- de Vries E, van de Poll-Franse LV, Louwman WJ, de Gruijl FR, Coebergh JWW. Predictions of skin cancer incidence in the Netherlands up to 2015. The British Journal of Dermatology. 2005;152(3):481–488. - PubMed
-
- Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American joint committee on cancer staging system for cutaneous melanoma. Journal of Clinical Oncology. 2001;19(16):3635–3648. - PubMed
-
- Reszka K, Jimbow K. Electron donor and acceptor properties of melanin pigments in the skin. In: Fuchs J, Packer L, editors. Oxidative Stress in Dermatology. New York, NY, USA: Marcel Dekker; 1993. pp. 287–320.
-
- Jimbow K, Iwashina T, Alena F, Yamada K, Pankovich J, Umemura T. Exploitation of pigment biosynthesis pathway as a selective chemotherapeutic approach for malignant melanoma. Journal of Investigative Dermatology. 1993;100(2):s231–s238. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

