Bisphosphonates and cancer: what opportunities from nanotechnology?
- PMID: 23533771
- PMCID: PMC3603225
- DOI: 10.1155/2013/637976
Bisphosphonates and cancer: what opportunities from nanotechnology?
Abstract
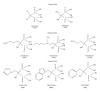
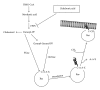
Bisphosphonates (BPs) are synthetic analogues of naturally occurring pyrophosphate compounds. They are used in clinical practice to inhibit bone resorption in bone metastases, osteoporosis, and Paget's disease. BPs induce apoptosis because they can be metabolically incorporated into nonhydrolyzable analogues of adenosine triphosphate. In addition, the nitrogen-containing BPs (N-BPs), second-generation BPs, act by inhibiting farnesyl diphosphate (FPP) synthase, a key enzyme of the mevalonate pathway. These molecules are able to induce apoptosis of a number of cancer cells in vitro. Moreover, antiangiogenic effect of BPs has also been reported. However, despite these promising properties, BPs rapidly accumulate into the bone, thus hampering their use to treat extraskeletal tumors. Nanotechnologies can represent an opportunity to limit BP accumulation into the bone, thus increasing drug level in extraskeletal sites of the body. Thus, nanocarriers encapsulating BPs can be used to target macrophages, to reduce angiogenesis, and to directly kill cancer cell. Moreover, nanocarriers can be conjugated with BPs to specifically deliver anticancer agent to bone tumors. This paper describes, in the first part, the state-of-art on the BPs, and, in the following part, the main studies in which nanotechnologies have been proposed to investigate new indications for BPs in cancer therapy.
Figures

References
-
- Ross JR, Saunders Y, Edmonds PM, et al. A systematic review of the role of bisphosphonates in metastatic disease. Health Technology Assessment. 2004;8(4):1–176. - PubMed
-
- Fleisch H, Russell RGG, Bisaz S, Casey PA, Mühlbauer RC. The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution of calcium phosphate in vitro and in vivo. Calcified Tissue Research. 1968;2(1):p. 10. - PubMed
-
- Russell RG. Bisphosphonates: the first 40 years. Bone. 2011;49(1):2–19. - PubMed
-
- Widler L, Jahnke W, Green JR. The chemistry of bisphosphonates: from antiscaling agents to clinical therapeutics. Anticancer Agents in Medicinals Chemistry. 2012;12(2):95–101. - PubMed
-
- Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(supplement 2):S150–S162. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

