Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration
- PMID: 23533946
- PMCID: PMC3606733
- DOI: 10.1155/2013/503725
Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration
Abstract
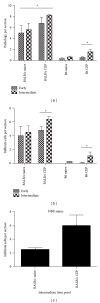
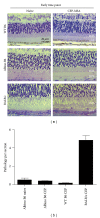
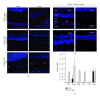
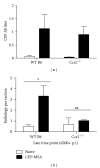
Age-related macular degeneration (AMD) is a major cause of blindness in the developed world. Oxidative stress and inflammation are implicated in AMD, but precise mechanisms remain poorly defined. Carboxyethylpyrrole (CEP) is an AMD-associated lipid peroxidation product. We previously demonstrated that mice immunized with CEP-modified albumin developed AMD-like degenerative changes in the outer retina. Here, we examined the kinetics of lesion development in immunized mice and the presence of macrophages within the interphotoreceptor matrix (IPM), between the retinal pigment epithelium and photoreceptor outer segments. We observed a significant and time-dependent increase in the number of macrophages in immunized mice relative to young age-matched controls prior to overt pathology. These changes were more pronounced in BALB/c mice than in C57BL/6 mice. Importantly, IPM-infiltrating macrophages were polarized toward the M1 phenotype but only in immunized mice. Moreover, when Ccr2-deficient mice were immunized, macrophages were not present in the IPM and no retinal lesions were observed, suggesting a deleterious role for these cells in our model. This work provides mechanistic evidence linking immune responses against oxidative damage with the presence of proinflammatory macrophages at sites of future AMD and experimentally demonstrates that manipulating immunity may be a target for modulating the development of AMD.
Figures

References
-
- Augood CA, Vingerling JR, De Jong PTVM, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Archives of Ophthalmology. 2006;124(4):529–535. - PubMed
-
- Javitt JC, Zhou Z, Maguire MG, Fine SL, Willke RJ. Incidence of exudative age-related macular degeneration among elderly Americans. Ophthalmology. 2003;110(8):1534–1539. - PubMed
-
- Bressler SB, Maguire MG, Bressler NM, Fine SL. Relationship of drusen and abnormalities of the retinal pigment epithelium to the prognosis of neovascular macular degeneration. Archives of Ophthalmology. 1990;108(10):1442–1447. - PubMed
-
- Sarks SH, Van Driel D, Maxwell L, Killingsworth M. Softening of drusen and subretinal neovascularization. Transactions of the Ophthalmological Societies of the United Kingdom. 1980;100(3):414–422. - PubMed
-
- Holz FG, Bellman C, Staudt S, Schütt F, Völcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Investigative Ophthalmology and Visual Science. 2001;42(5):1051–1056. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

