The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages
- PMID: 23533994
- PMCID: PMC3591180
- DOI: 10.1155/2013/187204
The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages
Abstract
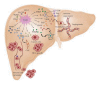
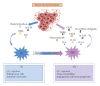
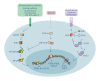
Hepatocellular carcinoma (HCC) is one of the most common and aggressive human cancers worldwide. HCC is an example of inflammation-related cancer and represents a paradigm of the relation occurring between tumor microenvironment and tumor development. Tumor-associated macrophages (TAMs) are a major component of leukocyte infiltrate of tumors and play a pivotal role in tumor progression of inflammation-related cancer, including HCC. Several studies indicate that, in the tumor microenvironment, TAMs acquire an M2-polarized phenotype and promote angiogenesis, metastasis, and suppression of adaptive immunity through the expression of cytokines, chemokines, growth factors, and matrix metalloproteases. Indeed, an established M2 macrophage population has been associated with poor prognosis in HCC. The molecular links that connect cancer cells and TAMs are not completely known, but recent studies have demonstrated that NF-κB, STAT-3, and HIF-1 signaling pathways play key roles in this crosstalk. In this paper, we discuss the current knowledge about the role of TAMs in HCC development, highlighting the role of TAM-derived cytokines, chemokines, and growth factors in the initiation and progression of liver cancer and outlining the signaling pathways involved in the interplay between cancer cells and TAMs.
Figures
References
-
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5(3):215–219. - PubMed
-
- Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Annals of the New York Academy of Sciences. 2009;1155:206–221. - PubMed
-
- Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Letters. 2011;305(2):250–262. - PubMed
-
- Budhu A, Xin WW. The role of cytokines in hepatocellular carcinoma. Journal of Leukocyte Biology. 2006;80(6):1197–1213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

