Rhinovirus wheezing illness and genetic risk of childhood-onset asthma
- PMID: 23534543
- PMCID: PMC3755952
- DOI: 10.1056/NEJMoa1211592
Rhinovirus wheezing illness and genetic risk of childhood-onset asthma
Abstract
Background: Both genetic variation at the 17q21 locus and virus-induced respiratory wheezing illnesses are associated with the development of asthma. Our aim was to determine the effects of these two factors on the risk of asthma in the Childhood Origins of Asthma (COAST) and the Copenhagen Prospective Study on Asthma in Childhood (COPSAC) birth cohorts.
Methods: We tested genotypes at the 17q21 locus for associations with asthma and with human rhinovirus (HRV) and respiratory syncytial virus (RSV) wheezing illnesses and tested for interactions between 17q21 genotypes and HRV and RSV wheezing illnesses with respect to the risk of asthma. Finally, we examined genotype-specific expression of 17q21 genes in unstimulated and HRV-stimulated peripheral-blood mononuclear cells (PBMCs).
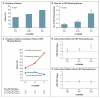
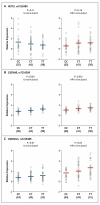
Results: The 17q21 variants were associated with HRV wheezing illnesses in early life, but not with RSV wheezing illnesses. The associations of 17q21 variants with asthma were restricted to children who had had HRV wheezing illnesses, resulting in a significant interaction effect with respect to the risk of asthma. Moreover, the expression levels of ORMDL3 and of GSDMB were significantly increased in HRV-stimulated PBMCs, as compared with unstimulated PBMCs. The expression of these genes was associated with 17q21 variants in both conditions, although the increase with exposure to HRV was not genotype-specific.
Conclusions: Variants at the 17q21 locus were associated with asthma in children who had had HRV wheezing illnesses and with expression of two genes at this locus. The expression levels of both genes increased in response to HRV stimulation, although the relative increase was not associated with the 17q21 genotypes. (Funded by the National Institutes of Health.).
Figures


Comment in
-
[Rhinovirus wheezing illness and genetic risk of childhood-onset asthma].Arch Argent Pediatr. 2013 Jul-Aug;111(4):366. Arch Argent Pediatr. 2013. PMID: 24066382 Spanish. No abstract available.
References
-
- Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–3. - PubMed
-
- Akhabir L, Sandford AJ. Genomewide association studies for discovery of genes involved in asthma. Respirology. 2011;16:396–406. - PubMed
-
- Bouzigon E, Corda E, Aschard H, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
