PKA: lessons learned after twenty years
- PMID: 23535202
- PMCID: PMC3763834
- DOI: 10.1016/j.bbapap.2013.03.007
PKA: lessons learned after twenty years
Abstract
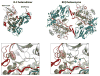
The first protein kinase structure, solved in 1991, revealed the fold that is shared by all members of the eukaryotic protein kinase superfamily and showed how the conserved sequence motifs cluster mostly around the active site. This structure of the PKA catalytic (C) subunit showed also how a single phosphate integrated the entire molecule. Since then the EPKs have become a major drug target, second only to the G-protein coupled receptors. Although PKA provided a mechanistic understanding of catalysis that continues to serve as a prototype for the family, by comparing many active and inactive kinases we subsequently discovered a hydrophobic spine architecture that is a characteristic feature of all active kinases. The ways in which the regulatory spine is dynamically assembled is the defining feature of each protein kinase. Protein kinases have thus evolved to be molecular switches, like the G-proteins, and unlike metabolic enzymes which have evolved to be efficient catalysis. PKA also shows how the dynamic tails surround the core and serve as essential regulatory elements. The phosphorylation sites in PKA, introduced both co- and post-translationally, are very stable. The resulting C-subunit is then packaged as an inhibited holoenzyme with cAMP-binding regulatory (R) subunits so that PKA activity is regulated exclusively by cAMP, not by the dynamic turnover of an activation loop phosphate. We could not understand activation and inhibition without seeing structures of R:C complexes; however, to appreciate the structural uniqueness of each R2:C2 holoenzyme required solving structures of tetrameric holoenzymes. It is these tetrameric holoenzymes that are localized to discrete sites in the cell, typically by A Kinase Anchoring Proteins where they create discrete foci for PKA signaling. Understanding these dynamic macromolecular complexes is the challenge that we now face. This article is part of a Special Issue entitled: Inhibitors of Protein Kinases (2012).
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
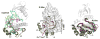



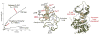

References
-
- Krebs EG, Graves DJ, Fischer EH. Factors affecting the activity of muscle phosphorylase B kinase. Journal of Biological Chemistry. 1959;234:2867–2873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

