Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon
- PMID: 23535335
- PMCID: PMC3645673
- DOI: 10.3390/ijms14046981
Melatonin and pancreatic islets: interrelationships between melatonin, insulin and glucagon
Abstract
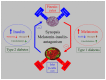
The pineal hormone melatonin exerts its influence in the periphery through activation of two specific trans-membrane receptors: MT1 and MT2. Both isoforms are expressed in the islet of Langerhans and are involved in the modulation of insulin secretion from β-cells and in glucagon secretion from α-cells. De-synchrony of receptor signaling may lead to the development of type 2 diabetes. This notion has recently been supported by genome-wide association studies identifying particularly the MT2 as a risk factor for this rapidly spreading metabolic disturbance. Since melatonin is secreted in a clearly diurnal fashion, it is safe to assume that it also has a diurnal impact on the blood-glucose-regulating function of the islet. This factor has hitherto been underestimated; the disruption of diurnal signaling within the islet may be one of the most important mechanisms leading to metabolic disturbances. The study of melatonin-insulin interactions in diabetic rat models has revealed an inverse relationship: an increase in melatonin levels leads to a down-regulation of insulin secretion and vice versa. Elucidation of the possible inverse interrelationship in man may open new avenues in the therapy of diabetes.
Figures

References
-
- Parhon C.I. Congrès d’Endocrinologie de Bucarest. 1939;I:187.
-
- Parhon C.I., Potop I., Felix E., Boeru V. Influenta epifisectomiei si a administrarii de extract epifisar asupra unor data metabolice privind mineralele (Ca, K, P, Si, Mg), lipidele, protidele si glucidele, la sobolanul alb adult. Stud. Cercet. Endocr. 1952;3:321–329.
-
- Milcou I., Nanu L., Marcean R. De l’existence d’une hormone hypoglycémiante épiphysaire synergique de l’insuline. Ann. Endocrinol. (Paris) 1957;18:612–620. - PubMed
-
- Milcu S.M. Role de l’épiphyse dans le metabolisme glucidique. J. Annu. Diabetol. Dieu. 1968;9:163–180. - PubMed
-
- Milcu S.M., Milcu I. Über ein hypoglykämisch wirkendes Hormon in der Zirbeldrüse. Medizinische. 1958;17:711–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

