Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent
- PMID: 23535460
- PMCID: PMC3652167
- DOI: 10.1152/ajpregu.00082.2013
Hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent
Abstract
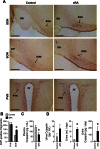
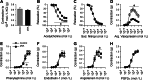
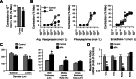
An indispensable role for the brain renin-angiotensin system (RAS) has been documented in most experimental animal models of hypertension. To identify the specific efferent pathway activated by the brain RAS that mediates hypertension, we examined the hypothesis that elevated arginine vasopressin (AVP) release is necessary for hypertension in a double-transgenic model of brain-specific RAS hyperactivity (the "sRA" mouse model). sRA mice experience elevated brain RAS activity due to human angiotensinogen expression plus neuron-specific human renin expression. Total daily loss of the 4-kDa AVP prosegment (copeptin) into urine was grossly elevated (≥8-fold). Immunohistochemical staining for AVP was increased in the supraoptic nucleus of sRA mice (~2-fold), but no quantitative difference in the paraventricular nucleus was observed. Chronic subcutaneous infusion of a nonselective AVP receptor antagonist conivaptan (YM-087, Vaprisol, 22 ng/h) or the V(2)-selective antagonist tolvaptan (OPC-41061, 22 ng/h) resulted in normalization of the baseline (~15 mmHg) hypertension in sRA mice. Abdominal aortas and second-order mesenteric arteries displayed AVP-specific desensitization, with minor or no changes in responses to phenylephrine and endothelin-1. Mesenteric arteries exhibited substantial reductions in V(1A) receptor mRNA, but no significant changes in V(2) receptor expression in kidney were observed. Chronic tolvaptan infusion also normalized the (5 mmol/l) hyponatremia of sRA mice. Together, these data support a major role for vasopressin in the hypertension of mice with brain-specific hyperactivity of the RAS and suggest a primary role of V(2) receptors.
Keywords: Vaprisol; antidiuretic hormone.
Figures


Comment in
-
Editorial Focus: the brain renin-angiotensin system and hypertension. Focus on: hypertension in mice with transgenic activation of the brain renin-angiotensin system is vasopressin dependent.Am J Physiol Regul Integr Comp Physiol. 2013 Aug 1;305(3):R173-4. doi: 10.1152/ajpregu.00272.2013. Epub 2013 Jun 5. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23739346 No abstract available.
References
-
- Aoyagi T, Koshimizu TA, Tanoue A. Vasopressin regulation of blood pressure and volume: findings from V1a receptor-deficient mice. Kidney Int 76: 1035–1039, 2009 - PubMed
-
- Argent NB, Burrell LM, Goodship TH, Wilkinson R, Baylis PH. Osmoregulation of thirst and vasopressin release in severe chronic renal failure. Kidney Int 39: 295–300, 1991 - PubMed
-
- Bakris G, Bursztyn M, Gavras I, Bresnahan M, Gavras H. Role of vasopressin in essential hypertension: racial differences. J Hypertens 15: 545–550, 1997 - PubMed
-
- Bonjour JP, Malvin RL. Stimulation of ADH release by the renin-angiotensin system. Am J Physiol 218: 1555–1559, 1970 - PubMed
-
- Burnatowska-Hledin M, Zeneberg A, Roulo A, Grobe J, Zhao P, Lelkes PI, Clare P, Barney C. Expression of VACM-1 protein in cultured rat adrenal endothelial cells is linked to the cell cycle. Endothelium 8: 49–63, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

