L74V increases the reverse transcriptase content of HIV-1 virions with non-nucleoside reverse transcriptase drug-resistant mutations L100I+K103N and K101E+G190S, which results in increased fitness
- PMID: 23535575
- PMCID: PMC3709637
- DOI: 10.1099/vir.0.050914-0
L74V increases the reverse transcriptase content of HIV-1 virions with non-nucleoside reverse transcriptase drug-resistant mutations L100I+K103N and K101E+G190S, which results in increased fitness
Abstract
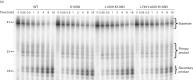
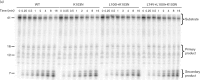
The fitness of non-nucleoside reverse transcriptase inhibitor (NNRTI) drug-resistant reverse transcriptase (RT) mutants of HIV-1 correlates with the amount of RT in the virions and the RNase H activity of the RT. We wanted to understand the mechanism by which secondary NNRTI-resistance mutations, L100I and K101E, and the nucleoside resistance mutation, L74V, alter the fitness of K103N and G190S viruses. We measured the amount of RT in virions and the polymerization and RNase H activities of mutant RTs compared to wild-type, K103N and G190S. We found that L100I, K101E and L74V did not change the polymerization or RNase H activities of K103N or G190S RTs. However, L100I and K101E reduced the amount of RT in the virions and subsequent addition of L74V restored RT levels back to those of G190S or K103N alone. We conclude that fitness changes caused by L100I, K101E and L74V derive from their effects on RT content.
Figures








Similar articles
-
Reduced fitness in cell culture of HIV-1 with nonnucleoside reverse transcriptase inhibitor-resistant mutations correlates with relative levels of reverse transcriptase content and RNase H activity in virions.J Virol. 2010 Sep;84(18):9377-89. doi: 10.1128/JVI.00618-10. Epub 2010 Jun 30. J Virol. 2010. PMID: 20592075 Free PMC article.
-
Reverse transcriptase backbone can alter the polymerization and RNase activities of non-nucleoside reverse transcriptase mutants K101E+G190S.J Gen Virol. 2013 Oct;94(Pt 10):2297-2308. doi: 10.1099/vir.0.054999-0. Epub 2013 Jun 26. J Gen Virol. 2013. PMID: 23804564 Free PMC article.
-
Relative replication fitness of efavirenz-resistant mutants of HIV-1: correlation with frequency during clinical therapy and evidence of compensation for the reduced fitness of K103N + L100I by the nucleoside resistance mutation L74V.Virology. 2006 Sep 15;353(1):184-92. doi: 10.1016/j.virol.2006.05.021. Epub 2006 Jun 21. Virology. 2006. PMID: 16797050 Free PMC article.
-
Structural and biochemical effects of human immunodeficiency virus mutants resistant to non-nucleoside reverse transcriptase inhibitors.Int J Biochem Cell Biol. 2004 Sep;36(9):1735-51. doi: 10.1016/j.biocel.2004.02.026. Int J Biochem Cell Biol. 2004. PMID: 15183341 Review.
-
HIV-1 reverse transcriptase connection subdomain mutations involved in resistance to approved non-nucleoside inhibitors.Antiviral Res. 2011 Nov;92(2):139-49. doi: 10.1016/j.antiviral.2011.08.020. Epub 2011 Aug 28. Antiviral Res. 2011. PMID: 21896288 Review.
Cited by
-
A spatio-temporal assessment of simian/human immunodeficiency virus (SHIV) evolution reveals a highly dynamic process within the host.PLoS Pathog. 2017 May 25;13(5):e1006358. doi: 10.1371/journal.ppat.1006358. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542550 Free PMC article.
-
Primer ID Informs Next-Generation Sequencing Platforms and Reveals Preexisting Drug Resistance Mutations in the HIV-1 Reverse Transcriptase Coding Domain.AIDS Res Hum Retroviruses. 2015 Jun;31(6):658-68. doi: 10.1089/AID.2014.0031. Epub 2015 Apr 2. AIDS Res Hum Retroviruses. 2015. PMID: 25748056 Free PMC article.
References
-
- Archer R. H., Dykes C., Gerondelis P., Lloyd A., Fay P., Reichman R. C., Bambara R. A., Demeter L. M. (2000). Mutants of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase resistant to nonnucleoside reverse transcriptase inhibitors demonstrate altered rates of RNase H cleavage that correlate with HIV-1 replication fitness in cell culture. J Virol 74, 8390–8401 10.1128/JVI.74.18.8390-8401.2000 - DOI - PMC - PubMed
-
- Bacheler L. T., Anton E. D., Kudish P., Baker D., Bunville J., Krakowski K., Bolling L., Aujay M., Wang X. V. & other authors (2000). Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrob Agents Chemother 44, 2475–2484 10.1128/AAC.44.9.2475-2484.2000 - DOI - PMC - PubMed
-
- Bacheler L., Jeffrey S., Hanna G., D’Aquila R., Wallace L., Logue K., Cordova B., Hertogs K., Larder B. & other authors (2001). Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J Virol 75, 4999–5008 10.1128/JVI.75.11.4999-5008.2001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

