Sulfhydration mediates neuroprotective actions of parkin
- PMID: 23535647
- PMCID: PMC3622945
- DOI: 10.1038/ncomms2623
Sulfhydration mediates neuroprotective actions of parkin
Abstract
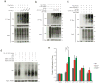
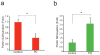
Increases in S-nitrosylation and inactivation of the neuroprotective ubiquitin E3 ligase, parkin, in the brains of patients with Parkinson's disease are thought to be pathogenic and suggest a possible mechanism linking parkin to sporadic Parkinson's disease. Here we demonstrate that physiologic modification of parkin by hydrogen sulfide, termed sulfhydration, enhances its catalytic activity. Sulfhydration sites are identified by mass spectrometry analysis and are investigated by site-directed mutagenesis. Parkin sulfhydration is markedly depleted in the brains of patients with Parkinson's disease, suggesting that this loss may be pathologic. This implies that hydrogen sulfide donors may be therapeutic.
Conflict of interest statement
Competing Financial Interests:
The authors declare no competing financial interests.
Figures


References
-
- Hatano T, Kubo S, Sato S, Hattori N. Pathogenesis of familial Parkinson’s disease: new insights based on monogenic forms of Parkinson’s disease. Journal of Neurochemistry. 2009;111:1075–1093. - PubMed
-
- Chung KKK, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin’s protective function. Science (New York, NY) 2004;304:1328–1331. - PubMed
-
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nature Reviews Drug Discovery. 2007;6:917–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

