Study protocol for a randomised controlled double-blinded trial of the dose-dependent efficacy and safety of primaquine for clearance of gametocytes in children with uncomplicated falciparum malaria in Uganda
- PMID: 23535703
- PMCID: PMC3612795
- DOI: 10.1136/bmjopen-2013-002759
Study protocol for a randomised controlled double-blinded trial of the dose-dependent efficacy and safety of primaquine for clearance of gametocytes in children with uncomplicated falciparum malaria in Uganda
Abstract
Objectives: For the purpose of blocking transmission of Plasmodium falciparum malaria from humans to mosquitoes, a single dose of primaquine is recommended by the WHO as an addition to artemisinin combination therapy. Primaquine clears gametocytes but causes dose-dependent haemolysis in individuals with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Evidence is needed to inform the optimal dosing of primaquine for malaria elimination programmes and for the purpose of interrupting the spread of artemisinin-resistant malaria. This study investigates the efficacy and safety of reducing doses of primaquine for clearance of gametocytes in participants with normal G6PD status.
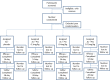
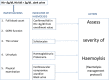
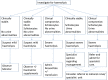
Methods and analysis: In this prospective, four-armed randomised placebo-controlled double-blinded trial, children aged 1-10 years, weighing over 10 kg, with haemoglobin ≥8 g/dl and uncomplicated P falciparum malaria are treated with artemether lumefantrine and randomised to receive a dose of primaquine (0.1, 0.4 or 0.75 mg base/kg) or placebo on the third day of treatment. Participants are followed up for 28 days. Gametocytaemia is measured by quantitative nucleic acid sequence-based analysis on days 0, 2, 3, 7, 10 and 14 with a primary endpoint of the number of days to gametocyte clearance in each treatment arm and secondarily the area under the curve of gametocyte density over time. Analysis is for non-inferiority of efficacy compared to the reference dose, 0.75 mg base/kg. Safety is assessed by pair-wise comparisons of the arithmetic mean (±SD) change in haemoglobin concentration per treatment arm and analysed for superiority to placebo and incidence of adverse events. Ethics and dissemination Approval was obtained from the ethical committees of Makerere University School of Medicine, the Ugandan National Council of Science and Technology and the London School of Hygiene and Tropical Medicine.
Results: These will be disseminated to inform malaria elimination policy, through peer-reviewed publication and academic presentations.
Figures
References
-
- Griffin JT, Hollingsworth TD, Okell LC, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med 2010;7 doi:10.1371/journal.pmed.1000324 (published Online First: Epub Date) - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous