Challenges and approaches for the development of safer immunomodulatory biologics
- PMID: 23535934
- PMCID: PMC7097261
- DOI: 10.1038/nrd3974
Challenges and approaches for the development of safer immunomodulatory biologics
Abstract
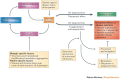
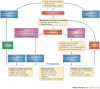
Immunomodulatory biologics, which render their therapeutic effects by modulating or harnessing immune responses, have proven their therapeutic utility in several complex conditions including cancer and autoimmune diseases. However, unwanted adverse reactions--including serious infections, malignancy, cytokine release syndrome, anaphylaxis and hypersensitivity as well as immunogenicity--pose a challenge to the development of new (and safer) immunomodulatory biologics. In this article, we assess the safety issues associated with immunomodulatory biologics and discuss the current approaches for predicting and mitigating adverse reactions associated with their use. We also outline how these approaches can inform the development of safer immunomodulatory biologics.
Conflict of interest statement
Lolke de Haan is an employee of Medimmune, Cambridge, UK.
James Green is an employee of Boehringer-Ingelheim, Sudbury, USA.
Jonathan Moggs is an employee of Novartis, Basel, Switzerland.
Jennifer Sims is an employee of Integrated Biologix, Basel, Switzerland.
Meena Subramanyam is an employee of Biogen Idec, Cambridge, Massachusetts, USA.
Marque Todd is an employee of Pfizer, California, USA.
Richard Weaver is an employee of Biologie Servier, Gidy, France.
All other authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

