Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study
- PMID: 23536134
- PMCID: PMC3644004
- DOI: 10.1001/jamapsychiatry.2013.736
Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study
Abstract
Importance: Genome-wide hypothesis-free discovery methods have identified loci that are associated with heavy smoking in adulthood. Research is needed to understand developmental processes that link newly discovered genetic risks with adult heavy smoking.
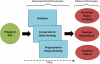
Objective: To test how genetic risks discovered in genome-wide association studies of adult smoking influence the developmental progression of smoking behavior from initiation through conversion to daily smoking, progression to heavy smoking, nicotine dependence, and struggles with cessation.
Design: A 38-year, prospective, longitudinal study of a representative birth cohort.
Setting: The Dunedin Multidisciplinary Health and Development Study of New Zealand.
Participants: The study included 1037 male and female participants.
Exposure: We assessed genetic risk with a multilocus genetic risk score. The genetic risk score was composed of single-nucleotide polymorphisms identified in 3 meta-analyses of genome-wide association studies of smoking quantity phenotypes.
Main outcomes and measures: Smoking initiation, conversion to daily smoking, progression to heavy smoking, nicotine dependence (Fagerström Test of Nicotine Dependence), and cessation difficulties were evaluated at 8 assessments spanning the ages of 11 to 38 years.
Results: Genetic risk score was unrelated to smoking initiation. However, individuals at higher genetic risk were more likely to convert to daily smoking as teenagers, progressed more rapidly from smoking initiation to heavy smoking, persisted longer in smoking heavily, developed nicotine dependence more frequently, were more reliant on smoking to cope with stress, and were more likely to fail in their cessation attempts. Further analysis revealed that 2 adolescent developmental phenotypes-early conversion to daily smoking and rapid progression to heavy smoking-mediated associations between the genetic risk score and mature phenotypes of persistent heavy smoking, nicotine dependence, and cessation failure. The genetic risk score predicted smoking risk over and above family history.
Conclusions and relevance: Initiatives that disrupt the developmental progression of smoking behavior among adolescents may mitigate genetic risks for developing adult smoking problems. Future genetic research may maximize discovery potential by focusing on smoking behavior soon after smoking initiation and by studying young smokers.
Figures







References
-
- Centers for Disease Control and Prevention (CDC) Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. - PubMed
-
- Current cigarette smoking prevalence among working adults--United States, 2004-2010. MMWR Morb Mortal Wkly Rep. 2011;60(38):1305–1309. - PubMed
Publication types
MeSH terms
Grants and funding
- T32-AG000029/AG/NIA NIH HHS/United States
- G0601483/MRC_/Medical Research Council/United Kingdom
- R01 MH077874/MH/NIMH NIH HHS/United States
- 1R36HS020524-01/HS/AHRQ HHS/United States
- P30 DA023026/DA/NIDA NIH HHS/United States
- MR/K00381X/1/MRC_/Medical Research Council/United Kingdom
- 1K05CA139871/CA/NCI NIH HHS/United States
- R01 AG032282/AG/NIA NIH HHS/United States
- L60 MD007327/MD/NIMHD NIH HHS/United States
- P2C HD065563/HD/NICHD NIH HHS/United States
- AG032282/AG/NIA NIH HHS/United States
- K05 CA139871/CA/NCI NIH HHS/United States
- R24 HD065563/HD/NICHD NIH HHS/United States
- R01 HL109031/HL/NHLBI NIH HHS/United States
- G0101483/MRC_/Medical Research Council/United Kingdom
- MH077874/MH/NIMH NIH HHS/United States
- P30DA023026/DA/NIDA NIH HHS/United States
- R36 HS020524/HS/AHRQ HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

