Alternative C-terminal helix orientation alters chemokine function: structure of the anti-angiogenic chemokine, CXCL4L1
- PMID: 23536183
- PMCID: PMC3650389
- DOI: 10.1074/jbc.M113.455329
Alternative C-terminal helix orientation alters chemokine function: structure of the anti-angiogenic chemokine, CXCL4L1
Abstract
Background: CXCL4L1 is a highly potent anti-angiogenic and anti-tumor chemokine, and its structural information is unknown.
Results: CXCL4L1 x-ray structure is determined, and it reveals a previously unrecognized chemokine structure adopting a novel C-terminal helix conformation.
Conclusion: The alternative helix conformation enhances the anti-angiogenic activity of CXCL4L1 by reducing the glycosaminoglycan binding ability.
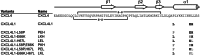
Significance: Chemokine C-terminal helix orientation is critical in regulating their functions. Chemokines, a subfamily of cytokines, are small, secreted proteins that mediate a variety of biological processes. Various chemokines adopt remarkable conserved tertiary structure comprising an anti-parallel β-sheet core domain followed by a C-terminal helix that packs onto the β-sheet. The conserved structural feature has been considered critical for chemokine function, including binding to cell surface receptor. The recently isolated variant, CXCL4L1, is a homologue of CXCL4 chemokine (or platelet factor 4) with potent anti-angiogenic activity and differed only in three amino acid residues of P58L, K66E, and L67H. In this study we show by x-ray structural determination that CXCL4L1 adopts a previously unrecognized structure at its C terminus. The orientation of the C-terminal helix protrudes into the aqueous space to expose the entire helix. The alternative helix orientation modifies the overall chemokine shape and surface properties. The L67H mutation is mainly responsible for the swing-out effect of the helix, whereas mutations of P58L and K66E only act secondarily. This is the first observation that reports an open conformation of the C-terminal helix in a chemokine. This change leads to a decrease of its glycosaminoglycan binding properties and to an enhancement of its anti-angiogenic and anti-tumor effects. This unique structure is recent in evolution and has allowed CXCL4L1 to gain novel functional properties.
Keywords: Angiogenesis; CXCL4L1; Chemokines; Heparin; Platelet Factor 4; Platelets; X-ray Crystallography.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

