Loss of interneuron LTD and attenuated pyramidal cell LTP in Trpv1 and Trpv3 KO mice
- PMID: 23536486
- PMCID: PMC3891370
- DOI: 10.1002/hipo.22125
Loss of interneuron LTD and attenuated pyramidal cell LTP in Trpv1 and Trpv3 KO mice
Abstract
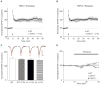
TRPV (transient receptor potential, vanilloid) channels are a family of nonselective cation channels that are activated by a wide variety of chemical and physical stimuli. TRPV1 channels are highly expressed in sensory neurons in the peripheral nervous system. However, a number of studies have also reported TRPV channels in the brain, though their functions are less well understood. In the hippocampus, the TRPV1 channel is a novel mediator of long-term depression (LTD) at excitatory synapses on interneurons. Here we tested the role of other TRPV channels in hippocampal synaptic plasticity, using hippocampal slices from Trpv1, Trpv3 and Trpv4 knockout (KO) mice. LTD at excitatory synapses on s. radiatum hippocampal interneurons was attenuated in slices from Trpv3 KO mice (as well as in Trpv1 KO mice as previously reported), but not in slices from Trpv4 KO mice. A previous study found that in hippocampal area CA1, slices from Trpv1 KO mice have reduced tetanus-induced long-term potentiation (LTP) following high-frequency stimulation; here we confirmed this and found a similar reduction in Trpv3 KO mice. We hypothesized that the loss of LTD at the excitatory synapses on local inhibitory interneurons caused the attenuated LTP in the mutants. Consistent with this idea, blocking GABAergic inhibition rescued LTP in slices from Trpv1 KO and Trpv3 KO mice. Our findings suggest a novel role for TRPV3 channels in synaptic plasticity and provide a possible mechanism by which TRPV1 and TRPV3 channels modulate hippocampal output.
Keywords: TRPV channel; TRPV1; TRPV3; synaptic plasticity.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

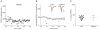


Similar articles
-
Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system.Neuropharmacology. 2011 Sep;61(4):730-8. doi: 10.1016/j.neuropharm.2011.05.018. Epub 2011 May 27. Neuropharmacology. 2011. PMID: 21645527
-
TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons.Neuron. 2008 Mar 13;57(5):746-59. doi: 10.1016/j.neuron.2007.12.027. Neuron. 2008. PMID: 18341994 Free PMC article.
-
C-type natriuretic peptide modulates bidirectional plasticity in hippocampal area CA1 in vitro.Neuroscience. 2010 Aug 11;169(1):8-22. doi: 10.1016/j.neuroscience.2010.04.064. Epub 2010 May 8. Neuroscience. 2010. PMID: 20438814
-
Aquaporin-4 water channels and synaptic plasticity in the hippocampus.Neurochem Int. 2013 Dec;63(7):702-11. doi: 10.1016/j.neuint.2013.05.003. Epub 2013 May 15. Neurochem Int. 2013. PMID: 23684954 Free PMC article. Review.
-
Transient receptor potential vanilloid (TRPV) channels: Basal properties and physiological potential.Gen Physiol Biophys. 2022 May;41(3):165-190. doi: 10.4149/gpb_2022016. Gen Physiol Biophys. 2022. PMID: 35615999 Review.
Cited by
-
Different ligands of the TRPV3 cation channel cause distinct conformational changes as revealed by intrinsic tryptophan fluorescence quenching.J Biol Chem. 2015 May 15;290(20):12964-74. doi: 10.1074/jbc.M114.628925. Epub 2015 Mar 31. J Biol Chem. 2015. PMID: 25829496 Free PMC article.
-
Differences in chloride gradients allow for three distinct types of synaptic modulation by endocannabinoids.J Neurophysiol. 2016 Aug 1;116(2):619-28. doi: 10.1152/jn.00235.2016. Epub 2016 May 25. J Neurophysiol. 2016. PMID: 27226449 Free PMC article.
-
TRPV3 in Drug Development.Pharmaceuticals (Basel). 2016 Sep 9;9(3):55. doi: 10.3390/ph9030055. Pharmaceuticals (Basel). 2016. PMID: 27618069 Free PMC article. Review.
-
Activity-Dependent Modulation of Tonic GABA Currents by Endocannabinoids in Hirudo verbana.Front Synaptic Neurosci. 2022 Mar 14;14:760330. doi: 10.3389/fnsyn.2022.760330. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35368247 Free PMC article.
-
No requirement of TRPV1 in long-term potentiation or long-term depression in the anterior cingulate cortex.Mol Brain. 2014 Apr 5;7:27. doi: 10.1186/1756-6606-7-27. Mol Brain. 2014. PMID: 24708859 Free PMC article.
References
-
- Bennion D, Jensen T, Walther C, Hamblin J, Wallmann A, Couch J, Blickenstaff J, Castle M, Dean L, Beckstead S, et al. Transient receptor potential vanilloid 1 agonists modulate hippocampal CA1 LTP via the GABAergic system. Neuropharmacology. 2011;61(4):730–8. - PubMed
-
- Caterina MJ. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R64–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

