Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M)
- PMID: 23536584
- PMCID: PMC3747937
- DOI: 10.2337/dc12-2006
Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M)
Abstract
Objective: To examine the efficacy and safety of lixisenatide (20 μg once daily, administered before the morning or evening meal) as add-on therapy in patients with type 2 diabetes insufficiently controlled with metformin alone.
Research design and methods: This was a 24-week, randomized, double-blind, placebo-controlled study in 680 patients with inadequately controlled type 2 diabetes (HbA1c 7-10% [53-86 mmol/mol]). Patients were randomized to lixisenatide morning (n = 255), lixisenatide evening (n = 255), placebo morning (n = 85), or placebo evening (n = 85) injections.
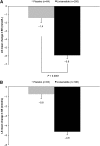
Results: Lixisenatide morning injection significantly reduced mean HbA1c versus combined placebo (mean change -0.9% [9.8 mmol/mol] vs. -0.4% [4.4 mmol/mol]; least squares [LS] mean difference vs. placebo -0.5% [5.5 mmol/mol], P < 0.0001). HbA1c was significantly reduced by lixisenatide evening injection (mean change -0.8% [8.7 mmol/mol] vs. -0.4% [4.4 mmol/mol]; LS mean difference -0.4% [4.4 mmol/mol], P < 0.0001). Lixisenatide morning injection significantly reduced 2-h postprandial glucose versus morning placebo (mean change -5.9 vs. -1.4 mmol/L; LS mean difference -4.5 mmol/L, P < 0.0001). LS mean difference in fasting plasma glucose was significant in both morning (-0.9 mmol/L, P < 0.0001) and evening (-0.6 mmol/L, P = 0.0046) groups versus placebo. Mean body weight decreased to a similar extent in all groups. Rates of adverse events were 69.4% in both lixisenatide groups and 60.0% in the placebo group. Rates for nausea and vomiting were 22.7 and 9.4% for lixisenatide morning and 21.2 and 13.3% for lixisenatide evening versus 7.6 and 2.9% for placebo, respectively. Symptomatic hypoglycemia occurred in 6, 13, and 1 patient for lixisenatide morning, evening, and placebo, respectively, with no severe episodes.
Conclusions: In patients with type 2 diabetes inadequately controlled on metformin, lixisenatide 20 μg once daily administered in the morning or evening significantly improved glycemic control, with a pronounced postprandial effect, and was well tolerated.
Trial registration: ClinicalTrials.gov NCT00712673.
Figures

References
-
- Aroda VR, Ratner R. The safety and tolerability of GLP-1 receptor agonists in the treatment of type 2 diabetes: a review. Diabetes Metab Res Rev 2011;27:528–542 - PubMed
-
- Campbell RK. Clarifying the role of incretin-based therapies in the treatment of type 2 diabetes mellitus. Clin Ther 2011;33:511–527 - PubMed
-
- Christensen M, Knop FK. Once-weekly GLP-1 agonists: how do they differ from exenatide and liraglutide? Curr Diab Rep 2010;10:124–132 - PubMed
-
- Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics)—preclinical and clinical results. Best Pract Res Clin Endocrinol Metab 2009;23:463–477 - PubMed
-
- Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists—available efficacy and safety data and perspectives for the future. Diabetes Obes Metab 2011;13:394–407 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

