Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria
- PMID: 23536632
- PMCID: PMC3633624
- DOI: 10.4049/jimmunol.1202672
Platelet induction of the acute-phase response is protective in murine experimental cerebral malaria
Abstract
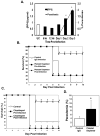
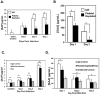
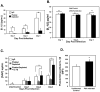
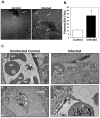
Platelets are most recognized as the cellular mediator of thrombosis, but they are increasingly appreciated for their immunomodulatory roles, including responses to Plasmodium infection. Platelet interactions with endothelial cells and leukocytes contribute significantly to the pathogenesis of experimental cerebral malaria (ECM). Recently, it has been suggested that platelets not only have an adverse role in cerebral malaria, but platelets may also be protective in animal models of uncomplicated malaria. We now demonstrate that these diverse and seemingly contradictory roles for platelets extend to cerebral malaria models and are dependent on the timing of platelet activation during infection. Our data show that platelets are activated very early in ECM and have a central role in initiation of the acute-phase response to blood-stage infection. Unlike platelet depletion or inhibition postinfection, preinfection platelet depletion or treatment with a platelet inhibitor is not protective. Additionally, we show that platelet-driven acute-phase responses have a major role in protecting mice from ECM by limiting parasite growth. Our data now suggest that platelets have a complex role in ECM pathogenesis: platelets help limit parasite growth early postinfection, but with continued platelet activation as the disease progresses, platelets contribute to ECM-associated inflammation.
Figures

References
-
- WHO. World Malaria Report: 2010. World Health Organization; Geneva, Switzerland: 2011.
-
- Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–466. - PubMed
-
- van der Hyde HC. A unified hypothesis for the genesis of cerebral malaria. Trends In Parasitology. 2006;22:503–508. - PubMed
-
- Wagner D. Platelets in Inflammation and Thrombosis. Arterioscler Thromb Vasc Biol. 2003;23:2131–2137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

