Role of fatty-acid synthesis in dendritic cell generation and function
- PMID: 23536633
- PMCID: PMC3633656
- DOI: 10.4049/jimmunol.1202312
Role of fatty-acid synthesis in dendritic cell generation and function
Abstract
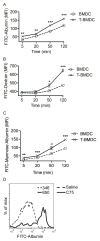
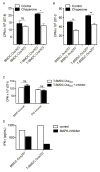
Dendritic cells (DC) are professional APCs that regulate innate and adaptive immunity. The role of fatty-acid synthesis in DC development and function is uncertain. We found that blockade of fatty-acid synthesis markedly decreases dendropoiesis in the liver and in primary and secondary lymphoid organs in mice. Human DC development from PBMC precursors was also diminished by blockade of fatty-acid synthesis. This was associated with higher rates of apoptosis in precursor cells and increased expression of cleaved caspase-3 and BCL-xL and downregulation of cyclin B1. Further, blockade of fatty-acid synthesis decreased DC expression of MHC class II, ICAM-1, B7-1, and B7-2 but increased their production of selected proinflammatory cytokines including IL-12 and MCP-1. Accordingly, inhibition of fatty-acid synthesis enhanced DC capacity to activate allogeneic as well as Ag-restricted CD4(+) and CD8(+) T cells and induce CTL responses. Further, blockade of fatty-acid synthesis increased DC expression of Notch ligands and enhanced their ability to activate NK cell immune phenotype and IFN-γ production. Because endoplasmic reticulum (ER) stress can augment the immunogenic function of APC, we postulated that this may account for the higher DC immunogenicity. We found that inhibition of fatty-acid synthesis resulted in elevated expression of numerous markers of ER stress in humans and mice and was associated with increased MAPK and Akt signaling. Further, lowering ER stress by 4-phenylbutyrate mitigated the enhanced immune stimulation associated with fatty-acid synthesis blockade. Our findings elucidate the role of fatty-acid synthesis in DC development and function and have implications to the design of DC vaccines for immunotherapy.
Figures






References
-
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. - PubMed
-
- Zeyda M, Saemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–14301. - PubMed
-
- Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, Knight SC, Padhya T, McCaffrey TV, McCaffrey JC, Antonia S, Fishman M, Ferris RL, Kagan VE, Gabrilovich DI. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. - PMC - PubMed
-
- Miller G, Lahrs S, Pillarisetty VG, Shah AB, DeMatteo RP. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and T-cell-mediated tumor protection. Cancer Res. 2002;62:5260–5266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

