Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2
- PMID: 23536638
- PMCID: PMC3740355
- DOI: 10.4049/jimmunol.1202857
Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2
Abstract
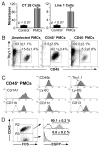
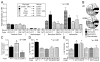
Fibrocytes are circulating, hematopoietic cells that express CD45 and Col1a1. They contribute to wound healing and several fibrosing disorders by mechanisms that are poorly understood. In this report, we demonstrate that fibrocytes predispose the lung to B16-F10 metastasis by recruiting Ly-6C(+) monocytes. To do so, we isolated fibrocytes expressing CD45, CD11b, CD13, and Col1a1 from the lungs of wild type (WT) and Ccr5(-/-) mice. WT but not Ccr5(-/-) fibrocytes increased the number of metastatic foci when injected into Ccr5(-/-) mice (73 ± 2 versus 32 ± 5; p < 0.001). This process was MMP9 dependent. Injection of WT enhanced GFP(+) fibrocytes also increased the number of Gr-1(Int), CD11b(+), and enhanced GFP(-) monocytes. Like premetastatic-niche monocytes, these recruited cells expressed Ly-6C, CD117, and CD45. The transfer of these cells into Ccr5(-/-) mice enhanced metastasis (90 ± 8 foci) compared with B cells (27 ± 2), immature dendritic cells (31 ± 6), or alveolar macrophages (28 ± 3; p < 0.05). WT and Ccl2(-/-) fibrocytes also stimulated Ccl2 expression in the lung by 2.07 ± 0.05- and 2.78 ± 0.36-fold compared with Ccr5(-/-) fibrocytes (1.0 ± 0.06; p < 0.05). Furthermore, WT fibrocytes did not increase Ly-6C(+) monocytes in Ccr2(-/-) mice and did not promote metastasis in either Ccr2(-/-) or Ccl2(-/-) mice. These data support our hypothesis that fibrocytes contribute to premetastatic conditioning by recruiting Ly-6C(+) monocytes in a chemokine-dependent process. This work links metastatic risk to conditions that mobilize fibrocytes, such as inflammation and wound repair.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



References
-
- Bucala R. Review Series – Inflammation & fibrosis fibrocytes and fibrosis. QJM. 2012;105:505–508. - PubMed
-
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. - PubMed
-
- Iqbal SA, Sidgwick GP, Bayat A. Identification of fibrocytes from mesenchymal stem cells in keloid tissue: a potential source of abnormal fibroblasts in keloid scarring. Arch Dermatol Res. 2012;304:665–671. - PubMed
-
- Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

