Postnatal development of synaptic properties of the GABAergic projection from the inferior colliculus to the auditory thalamus
- PMID: 23536710
- PMCID: PMC4459925
- DOI: 10.1152/jn.00021.2013
Postnatal development of synaptic properties of the GABAergic projection from the inferior colliculus to the auditory thalamus
Abstract
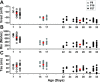
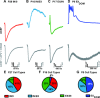
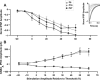
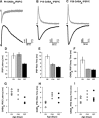
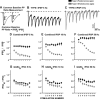
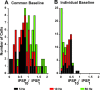
The development of auditory temporal processing is important for processing complex sounds as well as for acquiring reading and language skills. Neuronal properties and sound processing change dramatically in auditory cortex neurons after the onset of hearing. However, the development of the auditory thalamus or medial geniculate body (MGB) has not been well studied over this critical time window. Since synaptic inhibition has been shown to be crucial for auditory temporal processing, this study examined the development of a feedforward, GABAergic connection to the MGB from the inferior colliculus (IC), which is also the source of sensory glutamatergic inputs to the MGB. IC-MGB inhibition was studied using whole cell patch-clamp recordings from rat brain slices in current-clamp and voltage-clamp modes at three age groups: a prehearing group [postnatal day (P)7-P9], an immediate posthearing group (P15-P17), and a juvenile group (P22-P32) whose neuronal properties are largely mature. Membrane properties matured substantially across the ages studied. GABAA and GABAB inhibitory postsynaptic potentials were present at all ages and were similar in amplitude. Inhibitory postsynaptic potentials became faster to single shocks, showed less depression to train stimuli at 5 and 10 Hz, and were overall more efficacious in controlling excitability with age. Overall, IC-MGB inhibition becomes faster and more precise during a time period of rapid changes across the auditory system due to the codevelopment of membrane properties and synaptic properties.
Keywords: critical window; midbrain; paired pulse; tectothalamic; thalamocortical.
Figures




References
-
- Aghajanian GK, Rasmussen K. Intracellular studies in the facial nucleus illustrating a simple new method for obtaining viable motoneurons in adult rat brain slices. Synapse 3: 331–338, 1989. - PubMed
-
- Ammer JJ, Grothe B, Felmy F. Late postnatal development of intrinsic and synaptic properties promotes fast and precise signaling in the dorsal nucleus of the lateral lemniscus. J Neurophysiol 107: 1172–1185, 2012. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

