Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels
- PMID: 23536744
- PMCID: PMC3607213
- DOI: 10.1002/adfm.201200976
Integration of Self-Assembled Microvascular Networks with Microfabricated PEG-Based Hydrogels
Abstract
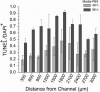
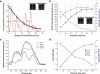
Despite tremendous efforts, tissue engineered constructs are restricted to thin, simple tissues sustained only by diffusion. The most significant barrier in tissue engineering is insufficient vascularization to deliver nutrients and metabolites during development in vitro and to facilitate rapid vascular integration in vivo. Tissue engineered constructs can be greatly improved by developing perfusable microvascular networks in vitro in order to provide transport that mimics native vascular organization and function. Here a microfluidic hydrogel is integrated with a self-assembling pro-vasculogenic co-culture in a strategy to perfuse microvascular networks in vitro. This approach allows for control over microvascular network self-assembly and employs an anastomotic interface for integration of self-assembled micro-vascular networks with fabricated microchannels. As a result, transport within the system shifts from simple diffusion to vessel supported convective transport and extra-vessel diffusion, thus improving overall mass transport properties. This work impacts the development of perfusable prevascularized tissues in vitro and ultimately tissue engineering applications in vivo.
Figures



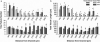

References
-
- Clark ER, Clark EL. Am. J. Anat. 1939;64:251–301.
-
- Carmeliet P, Jain RK. Nature. 2000;407:249–257. - PubMed
-
- Malda J, Rouwkema J, Martens DE, Le Comte EP, Kooy FK, Tramper J, van Blitterswijk CA, Riesle J. Biotechnol. Bioeng. 2004;86:9–18. - PubMed
-
- Rouwkema J, Rivron NC, van Blitterswijk CA. Trends Biotechnol. 2008;26:434–441. - PubMed
-
- Nicosia RF, Ottinetti A. In Vitro Cell Dev Biol. 1990;26:119–128. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
