Transcriptome analysis of spermatogenically regressed, recrudescent and active phase testis of seasonally breeding wall lizards Hemidactylus flaviviridis
- PMID: 23536792
- PMCID: PMC3594293
- DOI: 10.1371/journal.pone.0058276
Transcriptome analysis of spermatogenically regressed, recrudescent and active phase testis of seasonally breeding wall lizards Hemidactylus flaviviridis
Abstract
Background: Reptiles are phylogenically important group of organisms as mammals have evolved from them. Wall lizard testis exhibits clearly distinct morphology during various phases of a reproductive cycle making them an interesting model to study regulation of spermatogenesis. Studies on reptile spermatogenesis are negligible hence this study will prove to be an important resource.
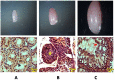
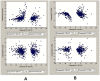
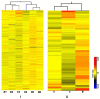
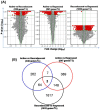
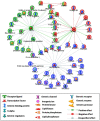
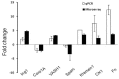
Methodology/principal findings: Histological analyses show complete regression of seminiferous tubules during regressed phase with retracted Sertoli cells and spermatognia. In the recrudescent phase, regressed testis regain cellular activity showing presence of normal Sertoli cells and developing germ cells. In the active phase, testis reaches up to its maximum size with enlarged seminiferous tubules and presence of sperm in seminiferous lumen. Total RNA extracted from whole testis of regressed, recrudescent and active phase of wall lizard was hybridized on Mouse Whole Genome 8×60 K format gene chip. Microarray data from regressed phase was deemed as control group. Microarray data were validated by assessing the expression of some selected genes using Quantitative Real-Time PCR. The genes prominently expressed in recrudescent and active phase testis are cytoskeleton organization GO 0005856, cell growth GO 0045927, GTpase regulator activity GO: 0030695, transcription GO: 0006352, apoptosis GO: 0006915 and many other biological processes. The genes showing higher expression in regressed phase belonged to functional categories such as negative regulation of macromolecule metabolic process GO: 0010605, negative regulation of gene expression GO: 0010629 and maintenance of stem cell niche GO: 0045165.
Conclusion/significance: This is the first exploratory study profiling transcriptome of three drastically different conditions of any reptilian testis. The genes expressed in the testis during regressed, recrudescent and active phase of reproductive cycle are in concordance with the testis morphology during these phases. This study will pave the way for deeper insight into regulation and evolution of gene regulatory mechanisms in spermatogenesis.
Conflict of interest statement
Figures


References
-
- Unsicker K, Burnstock G (1975) Myoid cells in the peritubular tissue (Lamina propria) of the reptilian testis. Cell Tissue Res 163: 545–560. - PubMed
-
- Reddy PR, Prasad MR (1970) Hormonal control of the maintenance of spermatogenesis and sexual segment in the Indian house lizard Hemidactylus flaviviridis Ruppell. Gen Comp Endocrinol 14: 15–24. - PubMed
-
- Rai U, Haider S (1995) Effects of cyproterone acetate on FSH and testosterone influenced spermatogenesis, steroidogenesis and epididymis in the Indian wall lizard, Hemidactylus flaviviridis (Ruppell). Eur J Morphol 33: 443–455. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

