Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319
- PMID: 23536797
- PMCID: PMC3594191
- DOI: 10.1371/journal.pone.0058575
Rational design of a fibroblast growth factor 21-based clinical candidate, LY2405319
Abstract
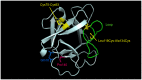
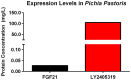
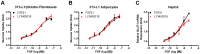
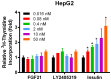
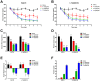
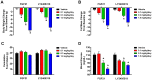
Fibroblast growth factor 21 is a novel hormonal regulator with the potential to treat a broad variety of metabolic abnormalities, such as type 2 diabetes, obesity, hepatic steatosis, and cardiovascular disease. Human recombinant wild type FGF21 (FGF21) has been shown to ameliorate metabolic disorders in rodents and non-human primates. However, development of FGF21 as a drug is challenging and requires re-engineering of its amino acid sequence to improve protein expression and formulation stability. Here we report the design and characterization of a novel FGF21 variant, LY2405319. To enable the development of a potential drug product with a once-daily dosing profile, in a preserved, multi-use formulation, an additional disulfide bond was introduced in FGF21 through Leu118Cys and Ala134Cys mutations. FGF21 was further optimized by deleting the four N-terminal amino acids, His-Pro-Ile-Pro (HPIP), which was subject to proteolytic cleavage. In addition, to eliminate an O-linked glycosylation site in yeast a Ser167Ala mutation was introduced, thus allowing large-scale, homogenous protein production in Pichia pastoris. Altogether re-engineering of FGF21 led to significant improvements in its biopharmaceutical properties. The impact of these changes was assessed in a panel of in vitro and in vivo assays, which confirmed that biological properties of LY2405319 were essentially identical to FGF21. Specifically, subcutaneous administration of LY2405319 in ob/ob and diet-induced obese (DIO) mice over 7-14 days resulted in a 25-50% lowering of plasma glucose coupled with a 10-30% reduction in body weight. Thus, LY2405319 exhibited all the biopharmaceutical and biological properties required for initiation of a clinical program designed to test the hypothesis that administration of exogenous FGF21 would result in effects on disease-related metabolic parameters in humans.
Conflict of interest statement
Figures

References
-
- Kharitonenkov A, Larsen P (2011) FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab 22: 81–86. - PubMed
-
- IDF (2011) IDF Diabetes Atlas, Fifth Edition. Brussels, Belgium: International Diabetes Federation.
-
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, et al. (2007) The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148: 774–781. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

