BRAHMA ATPase of the SWI/SNF chromatin remodeling complex acts as a positive regulator of gibberellin-mediated responses in arabidopsis
- PMID: 23536800
- PMCID: PMC3594165
- DOI: 10.1371/journal.pone.0058588
BRAHMA ATPase of the SWI/SNF chromatin remodeling complex acts as a positive regulator of gibberellin-mediated responses in arabidopsis
Abstract
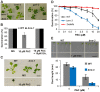
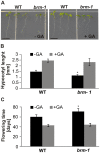
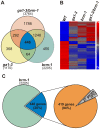
SWI/SNF chromatin remodeling complexes perform a pivotal function in the regulation of eukaryotic gene expression. Arabidopsis (Arabidopsis thaliana) mutants in major SWI/SNF subunits display embryo-lethal or dwarf phenotypes, indicating their critical role in molecular pathways controlling development and growth. As gibberellins (GA) are major positive regulators of plant growth, we wanted to establish whether there is a link between SWI/SNF and GA signaling in Arabidopsis. This study revealed that in brm-1 plants, depleted in SWI/SNF BRAHMA (BRM) ATPase, a number of GA-related phenotypic traits are GA-sensitive and that the loss of BRM results in markedly decreased level of endogenous bioactive GA. Transcriptional profiling of brm-1 and the GA biosynthesis mutant ga1-3, as well as the ga1-3/brm-1 double mutant demonstrated that BRM affects the expression of a large set of GA-responsive genes including genes responsible for GA biosynthesis and signaling. Furthermore, we found that BRM acts as an activator and directly associates with promoters of GA3ox1, a GA biosynthetic gene, and SCL3, implicated in positive regulation of the GA pathway. Many GA-responsive gene expression alterations in the brm-1 mutant are likely due to depleted levels of active GAs. However, the analysis of genetic interactions between BRM and the DELLA GA pathway repressors, revealed that BRM also acts on GA-responsive genes independently of its effect on GA level. Given the central position occupied by SWI/SNF complexes within regulatory networks controlling fundamental biological processes, the identification of diverse functional intersections of BRM with GA-dependent processes in this study suggests a role for SWI/SNF in facilitating crosstalk between GA-mediated regulation and other cellular pathways.
Conflict of interest statement
Figures



References
-
- Narlikar GJ, Fan H-Y, Kingston RE (2002) Cooperation between complexes that regulate chromatin structure and transcription. Cell 108: 475–487. - PubMed
-
- Clapier CR, Cairns BR (2009) The biology of chromatin remodeling complexes. Annu Rev Biochem 78: 273–304. - PubMed
-
- Martens JA, Winston F (2003) Recent advances in understanding chromatin remodeling by Swi/Snf complexes. Curr Opin Gen Dev 13: 136–142. - PubMed
-
- Yang X, Zaurin R, Beato M, Peterson CL (2007) Swi3p controls SWI/SNF assembly and ATP-dependent H2A-H2B displacement. Nat Struct Mol Biol 14: 540–547. - PubMed
-
- Jerzmanowski A (2007) SWI/SNF remodeling and linker histones in plants. Biochim Biophys Acta 1769: 330–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

