Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden
- PMID: 23537295
- PMCID: PMC3668985
- DOI: 10.1186/1476-4598-12-24
Short-term single treatment of chemotherapy results in the enrichment of ovarian cancer stem cell-like cells leading to an increased tumor burden
Abstract
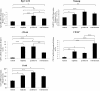
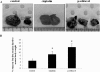
Over 80% of women diagnosed with advanced-stage ovarian cancer die as a result of disease recurrence due to failure of chemotherapy treatment. In this study, using two distinct ovarian cancer cell lines (epithelial OVCA 433 and mesenchymal HEY) we demonstrate enrichment in a population of cells with high expression of CSC markers at the protein and mRNA levels in response to cisplatin, paclitaxel and the combination of both. We also demonstrate a significant enhancement in the sphere forming abilities of ovarian cancer cells in response to chemotherapy drugs. The results of these in vitro findings are supported by in vivo mouse xenograft models in which intraperitoneal transplantation of cisplatin or paclitaxel-treated residual HEY cells generated significantly higher tumor burden compared to control untreated cells. Both the treated and untreated cells infiltrated the organs of the abdominal cavity. In addition, immunohistochemical studies on mouse tumors injected with cisplatin or paclitaxel treated residual cells displayed higher staining for the proliferative antigen Ki67, oncogeneic CA125, epithelial E-cadherin as well as cancer stem cell markers such as Oct4 and CD117, compared to mice injected with control untreated cells. These results suggest that a short-term single treatment of chemotherapy leaves residual cells that are enriched in CSC-like traits, resulting in an increased metastatic potential. The novel findings in this study are important in understanding the early molecular mechanisms by which chemoresistance and subsequent relapse may be triggered after the first line of chemotherapy treatment.
Figures






References
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. - DOI - PubMed
-
- Judson PL, Watson JM, Gehrig PA, Fowler WC Jr, Haskill JS. Cisplatin inhibits paclitaxel-induced apoptosis in cisplatin-resistant ovarian cancer cell lines: possible explanation for failure of combination therapy. Cancer Res. 1999;59:2425–2432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

