Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study
- PMID: 23537607
- PMCID: PMC3929944
- DOI: 10.1016/j.apmr.2013.03.009
Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St. Jude Lifetime Cohort Study
Abstract
Objectives: To ascertain prevalence of peripheral sensory and motor neuropathy, and to evaluate impairments in relation to function.
Design: St. Jude Lifetime Cohort Study, a clinical follow-up study designed to evaluate adverse late effects in adult survivors of childhood cancer.
Setting: A children's research hospital.
Participants: Eligibility required treatment for an extracranial solid malignancy between 1962 and 2002, age ≥ 18 years, ≥ 10 years postdiagnosis, and no history of cranial radiation. Survivors (N=531) were included in the evaluation with a median age of 32 years and a median time from diagnosis of 25 years.
Interventions: Not applicable.
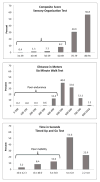
Main outcome measures: Primary exposure measures were cumulative doses of vinca-alkaloid and platinum-based chemotherapies. Survivors with scores ≥ 1 on the sensory subscale of the Modified Total Neuropathy Score were classified with prevalent sensory impairment. Those with sex-specific z scores of ≤-1.3 for dorsiflexion strength were classified with prevalent motor impairment. Participants completed the 6-minute walk test (endurance), the Timed Up & Go test (mobility), and the Sensory Organization Test (balance).
Results: The prevalence of sensory and motor impairment was 20% and 17.5%, respectively. Vinca-alkaloid exposure was associated with an increased risk of motor impairment (adjusted odds ratio [OR]=1.66; 95% confidence interval [CI], 1.04-2.64) without evidence for a dose response. Platinum exposure was associated with increased risk of sensory impairment (adjusted OR=1.62; 95% CI, .97-2.72) without evidence of a dose response. Sensory impairment was associated with poor endurance (OR=1.99; 95% CI, .99-4.0) and mobility (OR=1.65; 95% CI, .96-2.83).
Conclusions: Vincristine and cisplatin exposure may increase risk for long-term motor and sensory impairment, respectively. Survivors with sensory impairment are at increased risk for functional performance limitations.
Keywords: CI; Cisplatin; Modified Total Neuropathy Score; Neoplasms; OR; Peripheral nervous system diseases; Rehabilitation; SJLIFE; SOT; Sensory Organization Test; St. Jude Lifetime Cohort Study; TNS; Total Neuropathy Score; Vincristine; confidence interval; mTNS; odds ratio.
Copyright © 2013 American Congress of Rehabilitation Medicine. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. - PubMed
-
- Ross CJ, Visscher H, Rassekh SR, Castro-Pastrana LI, Shereck E, Carleton B, Hayden MR. Pharmacogenomics of serious adverse drug reactions in pediatric oncology. J Popul Ther Clin Pharmacol. 2011;18:e134–51. - PubMed
-
- Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–66. - PubMed
-
- Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical