Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis
- PMID: 23537713
- PMCID: PMC3652329
- DOI: 10.1016/j.nbd.2013.03.008
Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell death pathway, inhibits MT1 receptor loss and delays disease progression in a mouse model of amyotrophic lateral sclerosis
Abstract
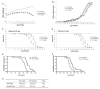
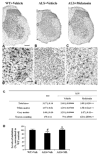
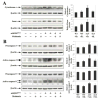
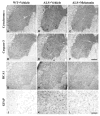
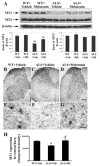
Caspase-mediated cell death contributes to the pathogenesis of motor neuron degeneration in the mutant SOD1(G93A) transgenic mouse model of amyotrophic lateral sclerosis (ALS), along with other factors such as inflammation and oxidative damage. By screening a drug library, we found that melatonin, a pineal hormone, inhibited cytochrome c release in purified mitochondria and prevented cell death in cultured neurons. In this study, we evaluated whether melatonin would slow disease progression in SOD1(G93A) mice. We demonstrate that melatonin significantly delayed disease onset, neurological deterioration and mortality in ALS mice. ALS-associated ventral horn atrophy and motor neuron death were also inhibited by melatonin treatment. Melatonin inhibited Rip2/caspase-1 pathway activation, blocked the release of mitochondrial cytochrome c, and reduced the overexpression and activation of caspase-3. Moreover, for the first time, we determined that disease progression was associated with the loss of both melatonin and the melatonin receptor 1A (MT1) in the spinal cord of ALS mice. These results demonstrate that melatonin is neuroprotective in transgenic ALS mice, and this protective effect is mediated through its effects on the caspase-mediated cell death pathway. Furthermore, our data suggest that melatonin and MT1 receptor loss may play a role in the pathological phenotype observed in ALS. The above observations indicate that melatonin and modulation of Rip2/caspase-1/cytochrome c or MT1 pathways may be promising therapeutic approaches for ALS.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

References
-
- Bensimon G, et al. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330:585–91. - PubMed
-
- Caballero B, et al. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in senescence-accelerated mice. J Pineal Res. 2008;45:302–11. - PubMed
-
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–19. - PubMed
-
- Drachman DB, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52:771–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

