Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer
- PMID: 23538216
- PMCID: PMC5528485
- DOI: 10.1016/j.molonc.2013.02.013
Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer
Abstract
Background: Enumeration of circulating tumor cells (CTC) from whole blood permits monitoring of patients with breast carcinoma. Analysis of apoptosis & Bcl-2 expression in CTC might add additional prognostic and predictive information. We estimated the degree of these markers in CTC from patients being treated for metastatic breast cancer.
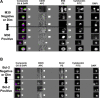
Methods: Eighty-three evaluable patients initiating a new therapy for metastatic breast cancer were enrolled. Whole blood was collected at baseline, at one of three short term time windows (24, 48, or 72 h) after initiating treatment, and at first follow-up (3-5 weeks). CTC were isolated, enumerated, and expression of M30 and Bcl2 was determined using the CellSearch(®) System.
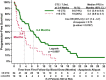
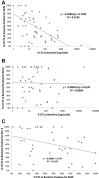
Results: At baseline, window, and 3-5 weeks post-treatment, 41/80 (51%), 40/80 (50%) and 21/75 (28%) patients had ≥5 CTC, respectively. At baseline, the proportion of CTC-apoptosis (M30) was inversely correlated with CTC number, and modestly inversely correlated with CTC-Bcl-2. As expected, higher CTC levels at baseline or first follow-up were associated with worse prognosis. Surprisingly, in patients with elevated CTC, higher levels of CTC-apoptosis were associated with worse prognosis, while higher CTC-Bcl-2 levels correlated with better outcomes.
Conclusions: CTC apoptosis and expression of Bcl-2 can be analytically determined in patients with metastatic breast cancer and may have biological and clinical implications. Characterization of CTC for these and other markers could further increase the utility of CTC monitoring patients in clinical investigations of new anti-neoplastic agents.
Copyright © 2013 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Albain, K.S. , Barlow, W.E. , Shak, S. , Hortobagyi, G.N. , Livingston, R.B. , Yeh, I.T. , Ravdin, P. , Bugarini, R. , Baehner, F.L. , Davidson, N.E. , Sledge, G.W. , Winer, E.P. , Hudis, C. , Ingle, J.N. , Perez, E.A. , Pritchard, K.I. , Shepherd, L. , Gralow, J.R. , Yoshizawa, C. , Allred, D.C. , Osborne, C.K. , Hayes, D.F. , 2010. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 11, 55–65. - PMC - PubMed
-
- Allard, J. , Hayes, D.F. , Repollet, M. , Rao, C. , Herman, M.L. , Matera, J. , Miller, M.C. , Terstappen, L.W. , 2003. A cellular preservative improves the specificity and yield of circulating tumor cells in carcinoma patients. Proc. Am. Soc. Clin. Oncol. 22, 866
-
- Allard, W.J. , Matera, J. , Miller, M.C. , Repollet, M. , Connelly, M.C. , Rao, C. , Tibbe, A.G. , Uhr, J.W. , Terstappen, L.W. , 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904. - PubMed
-
- Attard, G. , Crespo, M. , Lim, A.C. , Pope, L. , Zivi, A. , de Bono, J.S. , 2011. Reporting the capture efficiency of a filter-based microdevice: a CTC is not a CTC unless it is CD45 negative-letter. Clin. Cancer Res. 17, 3048–3049. Author reply 3050 - PubMed
-
- Attard, G. , Swennenhuis, J.F. , Olmos, D. , Reid, A.H. , Vickers, E. , A'Hern, R. , Levink, R. , Coumans, F. , Moreira, J. , Riisnaes, R. , Oommen, N.B. , Hawche, G. , Jameson, C. , Thompson, E. , Sipkema, R. , Carden, C.P. , Parker, C. , Dearnaley, D. , Kaye, S.B. , Cooper, C.S. , Molina, A. , Cox, M.E. , Terstappen, L.W. , de Bono, J.S. , 2009. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

