Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel
- PMID: 23538511
- PMCID: PMC3732325
- DOI: 10.4161/biom.24490
Enhancing retention and efficacy of cardiosphere-derived cells administered after myocardial infarction using a hyaluronan-gelatin hydrogel
Abstract
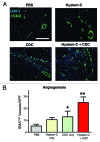
Cardiosphere-derived cells (CDCs) are under clinical development and are currently being tested in a clinical trial enrolling patients who have undergone a myocardial infarction. CDCs are presently administered via infusion into the infarct-related artery and have been shown in early clinical trials to be effective agents of myocardial regeneration. This review describes the administration of CDCs in a hyaluronan-gelatin hydrogel via myocardial injection and the subsequent improvements in therapeutic benefit seen in animal models. Development of a next generation therapy involving the combination of CDCs and hydrogel is discussed.
Keywords: ALLSTAR trial; CADUCEUS trial; cardiosphere-derived cells; hyaluronan-gelatin hydrogel; myocardial infarction.
Figures
References
-
- Smith RR, Kreke M, Malliaras K, Kanazawa H, Huang CH, Valle I, et al. Allogeneic cardiosphere-derived cells are safe and effective in a pig model of myocardial infarction. American Heart Association Annual Scientific Sessions 2012.
-
- Tseliou E, Pollan S, Malliaras K, Terrovitis J, Sun B, Galang G, et al. Allogeneic cardiospheres safely boost cardiac function and attenuate adverse remodeling after myocardial infarction in immunologically mismatched rat strains. J Am Coll Cardiol. 2013;61:1108–19. doi: 10.1016/j.jacc.2012.10.052. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
