The wobbler mouse, an ALS animal model
- PMID: 23539154
- PMCID: PMC3664746
- DOI: 10.1007/s00438-013-0741-0
The wobbler mouse, an ALS animal model
Abstract
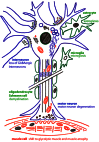
This review article is focused on the research progress made utilizing the wobbler mouse as animal model for human motor neuron diseases, especially the amyotrophic lateral sclerosis (ALS). The wobbler mouse develops progressive degeneration of upper and lower motor neurons and shows striking similarities to ALS. The cellular effects of the wobbler mutation, cellular transport defects, neurofilament aggregation, neuronal hyperexcitability and neuroinflammation closely resemble human ALS. Now, 57 years after the first report on the wobbler mouse we summarize the progress made in understanding the disease mechanism and testing various therapeutic approaches and discuss the relevance of these advances for human ALS. The identification of the causative mutation linking the wobbler mutation to a vesicle transport factor and the research focussed on the cellular basis and the therapeutic treatment of the wobbler motor neuron degeneration has shed new light on the molecular pathology of the disease and might contribute to the understanding the complexity of ALS.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

